Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity
- PMID: 11557981
- PMCID: PMC3900321
- DOI: 10.1038/35093109
Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity
Abstract
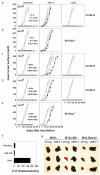
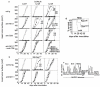
Natural killer (NK) cells attack many tumour cell lines, and are thought to have a critical role in anti-tumour immunity; however, the interaction between NK cells and tumour targets is poorly understood. The stimulatory lectin-like NKG2D receptor is expressed by NK cells, activated CD8+ T cells and by activated macrophages in mice. Several distinct cell-surface ligands that are related to class I major histocompatibility complex molecules have been identified, some of which are expressed at high levels by tumour cells but not by normal cells in adults. However, no direct evidence links the expression of these 'induced self' ligands with tumour cell rejection. Here we demonstrate that ectopic expression of the murine NKG2D ligands Rae1beta or H60 in several tumour cell lines results in potent rejection of the tumour cells by syngeneic mice. Rejection is mediated by NK cells and/or CD8+ T cells. The ligand-expressing tumour cells induce potent priming of cytotoxic T cells and sensitization of NK cells in vivo. Mice that are exposed to live or irradiated tumour cells expressing Rae1 or H60 are specifically immune to subsequent challenge with tumour cells that lack NKG2D ligands, suggesting application of the ligands in the design of tumour vaccines.
Figures



References
-
- Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H- 2-deficient lymphoma variants suggests alternative immune defense strategy. Nature. 1986;319:675–678. - PubMed
-
- Seaman W, Sleisenger M, Eriksson E, Koo G. Depletion of natural killer cells in mice by monoclonal antibody to NK-1.1. Reduction in host defense against malignancy without loss of cellular or humoral immunity. J. Immunol. 1987;138:4539–4544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

