Estrogen receptors: orchestrators of pleiotropic cellular responses
- PMID: 11559590
- PMCID: PMC1084040
- DOI: 10.1093/embo-reports/kve185
Estrogen receptors: orchestrators of pleiotropic cellular responses
Abstract
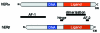
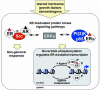
Estrogen receptors (ERs) orchestrate both transcriptional and non-genomic functions in response to estrogens, xenoestrogens and signals emanating from growth factor signalling pathways. The pleiotropic and tissue-specific effects of estrogens are likely to be mediated by the differential expression of distinct estrogen receptor subtypes (ERalpha and ERbeta) and their coregulators. The recent analysis of transcription complexes associated with estrogen-responsive promoters has revealed unexpected levels of complexity in the dynamics of ER-mediated transcription. Furthermore, a small fraction of ERs also appears to directly interact with components of the cytosolic signalling machinery. Analysis of the interrelationship between these distinct modes of ER action is likely to reveal novel aspects of estrogen signalling that will impact on nuclear receptor biology and human health.
Figures


References
-
- Bevan C. and Parker, M. (1999) The role of coactivators in steroid hormone action. Exp. Cell Res., 253, 349–356. - PubMed
-
- Burakov D., Wong, C.W., Rachez, C., Cheskis, B.J. and Freedman, L.P. (2000) Functional interactions between the estrogen receptor and DRIP205, a subunit of the heteromeric DRIP coactivator complex. J. Biol. Chem., 275, 20928–20934. - PubMed
-
- Campbell R.A., Bhat-Nakshatri, P., Patel, N.M., Constantinidou, D., Ali, S. and Nakshatri, H. (2001) PI3 kinase/AKT-mediated activation of estrogen receptor α: a new model for anti-estrogen resistance. J. Biol. Chem., 276, 9817–9824. - PubMed
-
- Chen D., Huang, S.M. and Stallcup, M.R. (2000a) Synergistic, p160 coactivator-dependent enhancement of estrogen receptor function by CARM1 and p300. J. Biol. Chem., 275, 40810–40816. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

