Olsalazine is not superior to placebo in maintaining remission of inactive Crohn's colitis and ileocolitis: a double blind, parallel, randomised, multicentre study
- PMID: 11559654
- PMCID: PMC1728458
- DOI: 10.1136/gut.49.4.552
Olsalazine is not superior to placebo in maintaining remission of inactive Crohn's colitis and ileocolitis: a double blind, parallel, randomised, multicentre study
Abstract
Background and aims: The benefit of 5-aminosalicylic acid therapy for maintenance of remission in Crohn's disease is controversial. The primary aim of this study was to evaluate the prophylactic properties of olsalazine in comparison with placebo for maintenance of remission in quiescent Crohn's colitis and/or ileocolitis.
Methods: In this randomised, double blind, parallel group study of olsalazine versus placebo, 328 patients with quiescent Crohn's colitis and/or ileocolitis were recruited. Treatment consisted of olsalazine 2.0 g daily or placebo for 52 weeks. The primary end point of efficacy was relapse, as defined by the Crohn's disease activity index (CDAI) and by clinical relapse. Laboratory and clinical disease activity indicators were also measured. Safety analysis consisted of documentation of adverse events and laboratory values.
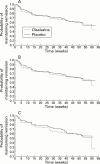
Results: No differences in the frequency of termination due to relapse or time to termination due to relapse were noted between the two treatment groups (olsalazine 48.5% v placebo 45%) for either colitis or ileocolitis. The failure rate, defined as not completing the study, was significantly higher in olsalazine treated patients compared with placebo treated patients for the overall population (colitis and/or ileocolitis: olsalazine 65.4% v 53.9%; p=0.038). Similar failure rates were seen for patients with colitis. A significantly higher percentage of olsalazine treated patients experienced adverse gastrointestinal events. Drug attributed adverse events were reported more frequently in the olsalazine treated group with gastrointestinal symptoms being causally related to olsalazine treatment (olsalazine 40.7% v placebo 26.9%; p=0.010). Back pain was reported significantly more often by the placebo treated group. However, serious medical events did not differ between the two groups. Adverse events led to more early withdrawals in the olsalazine treated group than in the placebo treated group; thus average time in the study for patients in the olsalazine treatment group was significantly shorter than that of patients in the placebo group.
Conclusions: Patients treated with olsalazine were more likely to terminate their participation in the trial than those taking placebo. This difference was not related to relapse of disease, as measured by CDAI and clinical measures, but rather was due to the development of intolerable adverse medical events of a non-serious nature related to the gastrointestinal tract. The gastrointestinal related events in the olsalazine treated group may be due to the difference in gastrointestinal status at baseline which favoured the placebo treatment group.
Figures
Comment in
-
Olsalazine was not better than placebo in maintaining remission in inactive Crohn disease.ACP J Club. 2002 May-Jun;136(3):92. doi: 10.7326/acpjc-2002-136-3-092. ACP J Club. 2002. PMID: 11985433 No abstract available.
Similar articles
-
Mesalazine (Mesasal/Claversal) 1.5 g b.d. vs. placebo in the maintenance of remission of patients with Crohn's disease.Aliment Pharmacol Ther. 1995 Dec;9(6):673-83. doi: 10.1111/j.1365-2036.1995.tb00438.x. Aliment Pharmacol Ther. 1995. PMID: 8824656 Clinical Trial.
-
Oral 4-aminosalicylic acid versus 5-aminosalicylic acid slow release tablets. Double blind, controlled pilot study in the maintenance treatment of Crohn's ileocolitis.Gut. 1994 Aug;35(8):1081-5. doi: 10.1136/gut.35.8.1081. Gut. 1994. PMID: 7926910 Free PMC article. Clinical Trial.
-
A randomized, double-blind, placebo-controlled trial of the oral mesalamine (5-ASA) preparation, Asacol, in the treatment of symptomatic Crohn's colitis and ileocolitis.J Clin Gastroenterol. 1994 Dec;19(4):278-82. doi: 10.1097/00004836-199412000-00003. J Clin Gastroenterol. 1994. PMID: 7876505 Clinical Trial.
-
The effect of mesalamine and nicotine in the treatment of inflammatory bowel disease.Ann Pharmacother. 1997 Jul-Aug;31(7-8):907-13. doi: 10.1177/106002809703100719. Ann Pharmacother. 1997. PMID: 9220055 Review.
-
Review article: maintenance treatment of Crohn's disease.Aliment Pharmacol Ther. 2003 Jun;17 Suppl 2:31-7. doi: 10.1046/j.1365-2036.17.s2.20.x. Aliment Pharmacol Ther. 2003. PMID: 12786610 Review.
Cited by
-
Aminosalicylates for induction of remission or response in Crohn's disease.Cochrane Database Syst Rev. 2016 Jul 3;7(7):CD008870. doi: 10.1002/14651858.CD008870.pub2. Cochrane Database Syst Rev. 2016. PMID: 27372735 Free PMC article.
-
Synthesis and evaluation of mutual azo prodrug of 5-aminosalicylic acid linked to 2-phenylbenzoxazole-2-yl-5-acetic acid in ulcerative colitis.Drug Des Devel Ther. 2013 Jul 31;7:691-8. doi: 10.2147/DDDT.S48636. eCollection 2013. Drug Des Devel Ther. 2013. PMID: 23983456 Free PMC article.
-
Oral 5-aminosalicylic acid for maintenance of surgically-induced remission in Crohn's disease.Cochrane Database Syst Rev. 2019 Jun 20;6(6):CD008414. doi: 10.1002/14651858.CD008414.pub3. Cochrane Database Syst Rev. 2019. PMID: 31220875 Free PMC article.
-
Crohn's disease.BMJ Clin Evid. 2011 Apr 27;2011:0416. BMJ Clin Evid. 2011. PMID: 21524318 Free PMC article.
-
European evidence based consensus on the diagnosis and management of Crohn's disease: current management.Gut. 2006 Mar;55 Suppl 1(Suppl 1):i16-35. doi: 10.1136/gut.2005.081950b. Gut. 2006. PMID: 16481629 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
