Gastrin and the neuropeptide PACAP evoke secretion from rat stomach histamine-containing (ECL) cells by stimulating influx of Ca2+ through different Ca2+ channels
- PMID: 11559765
- PMCID: PMC2278808
- DOI: 10.1111/j.1469-7793.2001.00663.x
Gastrin and the neuropeptide PACAP evoke secretion from rat stomach histamine-containing (ECL) cells by stimulating influx of Ca2+ through different Ca2+ channels
Abstract
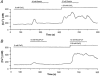
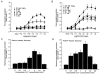
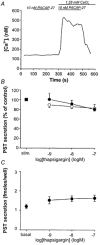
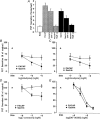
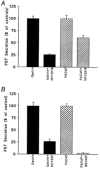
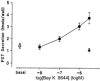
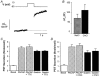
1. Gastrin and PACAP stimulate secretion of histamine and pancreastatin from isolated rat stomach ECL cells. We have examined whether or not secretion depends on the free cytosolic Ca2+ concentration ([Ca2+]i) and the pathways by which gastrin and PACAP elevate [Ca2+]i. Secretion was monitored by radioimmunoassay of pancreastatin and changes in [Ca2+]i by video imaging. The patch clamp technique was used to record whole-cell currents and membrane capacitance (reflecting exocytosis). 2. In the presence of 2 mM extracellular Ca2+, gastrin and PACAP induced secretion and raised [Ca2+]i. Without extracellular Ca2+ (or in the presence of La3+) no secretion occurred. The extracellular Ca2+ concentration required to stimulate secretion was 10 times higher for gastrin than for PACAP. Depletion of intracellular Ca2+ pools by thapsigargin had no effect on the capacity of gastrin and PACAP to stimulate secretion. 3. Gastrin-evoked secretion was inhibited 60-80 % by L-type channel blockers and 40 % by the N-type channel blocker omega-conotoxin GVIA. Combining L-type and N-type channel blockers did not result in greater inhibition than L-type channel blockers alone. Whole-cell patch clamp measurements confirmed that the ECL cells are equipped with voltage-dependent inward Ca2+ currents. A 500 ms depolarising pulse from -60 mV to +10 mV which maximally opened these channels resulted in an increase in membrane capacitance of 100 fF reflecting exocytosis of secretory vesicles. 4. PACAP-evoked secretion was reduced 40 % by L-type channel blockers but was not influenced by inhibition of N-type channels. SKF 96365, a blocker of both L-type and receptor-operated Ca2+ channels, inhibited PACAP-evoked secretion by 85 %. Combining L-type channel blockade with SKF 96365 abolished PACAP-evoked secretion. 5. The results indicate that gastrin- and PACAP-evoked secretion depends on Ca2+ entry and not on mobilisation of intracellular Ca2+. While gastrin stimulates secretion via voltage-dependent L-type and N-type Ca2+ channels, PACAP acts via L-type and receptor-operated Ca2+ channels.
Figures



References
-
- Berridge MJ, Irvine RF. Inositol trisphosphate: a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. - PubMed
-
- Bufler J, Choi GC, Francke C, Schepp W, Prinz C. Voltage-gated Ca2+ currents in rat gastric enterochromaffin-like cells. American Journal of Physiology. 1998;274:C424–429. - PubMed
-
- Burgoyne RD. Control of exocytosis in adrenal chromaffin cells. Biochimica et Biophysica Acta. 1991;1071:174–202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

