Calmodulin kinase and a calmodulin-binding 'IQ' domain facilitate L-type Ca2+ current in rabbit ventricular myocytes by a common mechanism
- PMID: 11559766
- PMCID: PMC2278813
- DOI: 10.1111/j.1469-7793.2001.t01-1-00679.x
Calmodulin kinase and a calmodulin-binding 'IQ' domain facilitate L-type Ca2+ current in rabbit ventricular myocytes by a common mechanism
Retraction in
-
Retraction: Calmodulin kinase and a calmodulin-binding ’IQ’ domain facilitate L-type Ca2+ current in rabbit ventricular myocytes by a common mechanism.J Physiol. 2015 May 1;593(9):2249. doi: 10.1113/JP270441. J Physiol. 2015. PMID: 26120650 Free PMC article. No abstract available.
Abstract
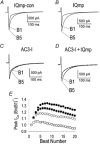
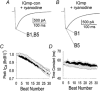
1. Ca2+-calmodulin-dependent protein kinase II (CaMK) and a calmodulin (CaM)-binding 'IQ' domain (IQ) are both implicated in Ca2+-dependent regulation of L-type Ca2+ current (I(Ca)). We used an IQ-mimetic peptide (IQmp), under conditions in which CaMK activity was controlled, to test the relationship between these CaM-activated signalling elements in the regulation of L-type Ca2+ channels (LTCCs) and I(Ca) in rabbit ventricular myocytes. 2. A specific CaMK inhibitory peptide nearly abolished I(Ca) facilitation, but the facilitation was 'rescued' by cell dialysis with IQmp. 3. IQmp significantly enhanced I(Ca) facilitation and slowed the fast component of I(Ca) inactivation, compared with an inactive control peptide. Neither effect could be elicited by a more avid CaM-binding peptide, suggesting that generalized CaM buffering did not account for the effects of IQmp. 4. I(Ca) facilitation was abolished and the fast component of inactivation eliminated by ryanodine, caffeine or thapsigargin, suggesting that the sarcoplasmic reticulum (SR) is an important source of Ca2+ for I(Ca) facilitation and inactivation. IQmp did not restore I(Ca) facilitation under these conditions. 5. Engineered Ca2+-independent CaMK and IQmp each markedly increased LTCC open probability (P(o)) in excised cell membrane patches. The LTCC P(o) increases with CaMK and IQmp were non-additive, suggesting that CaMK and IQmp are components of a shared signalling pathway. 6. Both CaMK and IQmp induced a modal gating shift in LTCCs that favoured prolonged openings, indicating that CaMK and IQmp affect LTCCs through a common biophysical mechanism. 7. These findings support the hypothesis that CaMK is required for physiological I(Ca) facilitation in cardiac myocytes. Both CaMK and IQmp were able to induce a modal gating shift in LTCCs, suggesting that each of these signalling elements is important for Ca2+-CaM-dependent LTCC facilitation in cardiac myocytes.
Figures
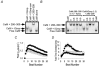


Comment in
-
Findings of research misconduct.NIH Guide Grants Contracts (Bethesda). 2014 Dec 5:NOT-OD-15-031. NIH Guide Grants Contracts (Bethesda). 2014. PMID: 25528784 Free PMC article. No abstract available.
-
Findings of Research Misconduct.Fed Regist. 2014 Nov 25;79(227):70187-70188. Fed Regist. 2014. PMID: 27737250 Free PMC article. No abstract available.
References
-
- Anderson ME, Braun AP, Schulman H, Premack BA. Multifunctional Ca2+/calmodulin-dependent protein kinase mediates Ca2+-induced enhancement of the L-type Ca2+ current in rabbit ventricular myocytes. Circulation Research. 1994;75:854–861. - PubMed
-
- Bers DM, Patton CW, Nuccitelli R. A practical guide to the preparation of Ca2+ buffers. Methods in Cell Biology. 1994;40:3–29. - PubMed
-
- Chao SH, Suzuki Y, Zysk JR, Cheung WY. Activation of calmodulin by various metal cations as a function of ionic radius. Molecular Pharmacology. 1984;26:75–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

