Nitric oxide is required for the induction and heterosynaptic spread of long-term potentiation in rat cerebellar slices
- PMID: 11559778
- PMCID: PMC2278807
- DOI: 10.1111/j.1469-7793.2001.t01-1-00825.x
Nitric oxide is required for the induction and heterosynaptic spread of long-term potentiation in rat cerebellar slices
Abstract
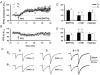
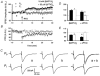
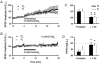
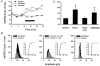
1. In the cerebellar cortex, brief, 8 Hz activation of parallel fibres (PFs) induces a cyclic adenosine 3'5'-monophosphate (cAMP) and protein kinase A (PKA)-dependent form of long-term potentiation between PFs and Purkinje cells. 2. With 10 mM BAPTA in the recording pipette, potentiation evoked by raised frequency stimulation (RFS) to one of two, synaptically independent PF inputs to the same Purkinje cell did not remain input specific but consistently spread to synapses that did not receive RFS, up to the maximum distance tested of 168 microm. 3. LTP at activated and non-activated sites was accompanied by a decrease in paired pulse facilitation (PPF). The PKA inhibitor H-89 blocked both of these effects. Inhibition of nitric oxide synthase (NOS), either by 7-nitro-indazole (7-NI) or N (G)-nitro-L-arginine methyl ester (L-NAME), completely prevented heterosynaptic potentiation and associated reduction in PPF. LTP at distant synapses was selectively prevented by the nitric oxide scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO). Inhibition of soluble guanylate cyclase or protein kinase G had no effect on either pathway. 4. Synaptic potentiation at PF-PC synapses, induced by the adenylate cyclase activator forskolin, was also prevented by inhibition of NOS. Forskolin-induced increases in mEPSC frequency were similarly prevented by NOS inhibition and mimicked by the NO donor spermine NONOate. 5. These results are consistent with the notion that heterosynaptic potentiation is of pre-synaptic origin and dependent upon activation of cAMP/PKA and NO. Moreover, they suggest that cAMP/PKA activation stimulates NO production and this diffusible messenger facilitates pre-synaptic transmitter release at synapses within a radius of upwards of 150 microm, through a mechanism that does not involve cGMP.
Figures
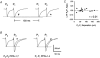
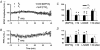
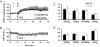
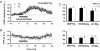

References
-
- Ahn S, Ginty DD, Linden DJ. A late phase of cerebellar long-term depression requires activation of CaMKIV and CREB. Neuron. 1999;23:559–568. - PubMed
-
- Anderson WW, Collingridge GL. A data acquisition program for on-line analysis of long-term potentiation and long-term depression. Society for Neuroscience Abstracts. 1999;23:665. - PubMed
-
- Arancio O, Kiebler M, Lee CJ, Levram V, Tsien RY, Kandel ER, Hawkins RD. Nitric-oxide acts directly in the presynaptic neuron to produce long-term potentiation in cultured hippocampal-neurons. Cell. 1996;87:1025–1035. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

