Slowly conducting afferents activated by innocuous low temperature in human skin
- PMID: 11559780
- PMCID: PMC2278822
- DOI: 10.1111/j.1469-7793.2001.t01-1-00855.x
Slowly conducting afferents activated by innocuous low temperature in human skin
Abstract
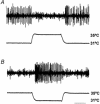
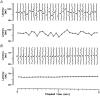
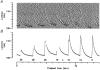
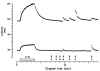
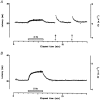
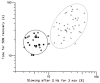
1. Microneurography was used to search for primary afferents responsive to innocuous low temperature in human nerves supplying the hairy skin of the hand or foot. Eighteen units were identified as cold-specific units: they displayed a steady-state discharge at skin temperatures in the range 28-30 degrees C, they were sensitive to small changes in temperature, and they responded vigorously when a cool metal probe touched their receptive fields (RFs). They were insensitive to mechanical stimuli and sympathetic activation. Their RFs comprised one, or at most two, spots less than 5 mm in diameter. 2. Nine units were characterised in detail by a series of 10 s cooling and warming pulses from a holding temperature of 35 degrees C. The threshold temperature for activation by cooling was 29.4 +/- 2.0 degrees C (mean +/- S.D.). Adaptation of the responses to supra-threshold cooling pulses was partial: mean peak and plateau firing rates were maximal on steps to 15 degrees C (35.9 and 19.9 impulses x s(-1), respectively). Three of these units also displayed a paradoxical response to warming, with a mean threshold of 42.3 degrees C. 3. Sixteen of the eighteen cold-specific units were also studied by electrical stimulation of their RFs. They conducted in the velocity range 0.8-3.0 m x s(-1). When stimulated at 2 Hz, their latency increased according to a characteristic time course, reaching a plateau within 3 min (mean slowing (+/- S.D.) 5.2 +/- 1.1 %) and recovering quickly (50 % recovery in 17.8 +/- 4.5 s). 4. To reconcile these findings with previous studies of reaction times and the effects of nerve compression on sensation, it is concluded that either human cold-specific afferent fibres are incompletely myelinated 'BC' fibres, or else there are C as well as A(delta) cold fibres, with the C fibre group contributing little to sensation.
Figures




References
-
- Adriaensen H, Gybels J, Handwerker HO, Van Hees J. Response properties of thin myelinated (A-δ) fibers in human skin nerves. Journal of Neurophysiology. 1983;49:111–122. - PubMed
-
- Darian-Smith I. Thermal sensibility. In: Darian-Smith I, editor. Handbook of Physiology, section 1, The Nervous System, Sensory Processes. Vol. 3. Bethesda, MD, USA: American Physiological Society; 1984. pp. 879–913. part 2.
-
- Darian-Smith I, Johnson KO, Dykes R. Cold’ fibre population innervating palmar and digital skin of the monkey: responses to cooling pulses. Journal of Neurophysiology. 1973;36:325–346. - PubMed
-
- Dodt E, Zotterman Y. The discharge of specific cold fibres at high temperatures (the paradoxical cold) Acta Physiologica Scandinavica. 1952;26:358–365. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

