Regression of established human papillomavirus type 16 (HPV-16) immortalized tumors in vivo by vaccinia viruses expressing different forms of HPV-16 E7 correlates with enhanced CD8(+) T-cell responses that home to the tumor site
- PMID: 11559797
- PMCID: PMC114536
- DOI: 10.1128/JVI.75.20.9654-9664.2001
Regression of established human papillomavirus type 16 (HPV-16) immortalized tumors in vivo by vaccinia viruses expressing different forms of HPV-16 E7 correlates with enhanced CD8(+) T-cell responses that home to the tumor site
Abstract
Using vaccinia virus as a live vector, we show that the expression of human papillomavirus type 16 (HPV-16) E7 fused to a nonhemolytic portion of the Listeria monocytogenes virulence factor, listeriolysin O (LLO), induces an immune response that causes the regression of established HPV-16 immortalized tumors in C57BL/6 mice. The vaccinia virus construct expressing LLO fused to E7 (VacLLOE7) was compared with two previously described vaccinia virus constructs: one that expresses unmodified E7 (VacE7) and another that expresses E7 in a form designed to direct it to intracellular lysosomal compartments and improve major histocompatibility complex class II-restricted responses (VacSigE7LAMP-1). C57BL/6 mice bearing established HPV-16 immortalized tumors of 5 or 8 mm were treated with each of these vaccines. Fifty percent of the mice treated with VacLLOE7 remained tumor free 2 months after tumor inoculation, whereas 12 to 25% of the mice were tumor free after treatment with VacSigE7LAMP-1 (depending on the size of the tumor). No mice were tumor free in the group given VacE7. Compared to VacE7, VacSigE7LAMP-1 and VacLLOE7 resulted in increased numbers of H2-D(b)-specific tetramer-positive CD8(+) T cells in mouse spleens that produced gamma interferon and tumor necrosis factor alpha upon stimulation with RAHYNIVTF peptide. In addition, the highest frequency of tetramer-positive T cells was seen in the tumor sites of mice treated with VacLLOE7. An increased efficiency of E7-specific lysis by splenocytes from mice immunized with VacLLOE7 was also observed. These results indicate that the fusion of E7 with LLO not only enhances antitumor therapy by improving the tumoricidal function of E7-specific CD8(+) T cells but may also increase the number of antigen-specific CD8(+) T cells in the tumor, the principle site of antigen expression.
Figures

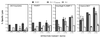





Similar articles
-
Antigen-specific immunotherapy of cervical and ovarian cancer.Immunol Rev. 2008 Apr;222:43-69. doi: 10.1111/j.1600-065X.2008.00622.x. Immunol Rev. 2008. PMID: 18363994 Free PMC article. Review.
-
Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16.J Immunol. 2001 Dec 1;167(11):6471-9. doi: 10.4049/jimmunol.167.11.6471. J Immunol. 2001. PMID: 11714814
-
Recombinant Listeria vaccines containing PEST sequences are potent immune adjuvants for the tumor-associated antigen human papillomavirus-16 E7.Cancer Res. 2004 Dec 15;64(24):8821-5. doi: 10.1158/0008-5472.CAN-04-1958. Cancer Res. 2004. PMID: 15604239
-
Boosting with recombinant vaccinia increases HPV-16 E7-specific T cell precursor frequencies of HPV-16 E7-expressing DNA vaccines.Vaccine. 2000 Apr 3;18(19):2015-22. doi: 10.1016/s0264-410x(99)00528-9. Vaccine. 2000. PMID: 10706963
-
Human papillomavirus genotype 16 vaccines for cervical cancer prophylaxis and treatment.Curr Opin Oncol. 2000 Sep;12(5):466-73. doi: 10.1097/00001622-200009000-00014. Curr Opin Oncol. 2000. PMID: 10975555 Review.
Cited by
-
Listeria monocytogenes-based antibiotic resistance gene-free antigen delivery system applicable to other bacterial vectors and DNA vaccines.Infect Immun. 2004 Nov;72(11):6418-25. doi: 10.1128/IAI.72.11.6418-6425.2004. Infect Immun. 2004. PMID: 15501772 Free PMC article.
-
Toll-like receptor agonist imiquimod facilitates antigen-specific CD8+ T-cell accumulation in the genital tract leading to tumor control through IFNγ.Clin Cancer Res. 2014 Nov 1;20(21):5456-67. doi: 10.1158/1078-0432.CCR-14-0344. Epub 2014 Jun 3. Clin Cancer Res. 2014. PMID: 24893628 Free PMC article.
-
Antigen-specific immunotherapy of cervical and ovarian cancer.Immunol Rev. 2008 Apr;222:43-69. doi: 10.1111/j.1600-065X.2008.00622.x. Immunol Rev. 2008. PMID: 18363994 Free PMC article. Review.
-
Ubiquitin-like Molecule ISG15 Acts as an Immune Adjuvant to Enhance Antigen-specific CD8 T-cell Tumor Immunity.Mol Ther. 2015 Oct;23(10):1653-62. doi: 10.1038/mt.2015.120. Epub 2015 Jun 30. Mol Ther. 2015. PMID: 26122932 Free PMC article.
-
Suppression of antitumour protective cytotoxic T lymphocyte responses to a human papillomavirus 16 E7 DNA vaccine by coinjection of interleukin-12 complementary DNA: involvement of nitric oxide in immune suppression.Immunology. 2009 Sep;128(1 Suppl):e707-17. doi: 10.1111/j.1365-2567.2009.03068.x. Epub 2009 Feb 9. Immunology. 2009. PMID: 19740332 Free PMC article.
References
-
- Boehm U, Klamp T, Groot M, Howard J C. Cellular responses to interferon-γ. Annu Rev Immunol. 1997;15:749–795. - PubMed
-
- Brodsky J L. Post-translational protein translation: not all HSC70s are created equal. Trends Biochem Sci. 1996;21:122–126. - PubMed
-
- Busch D H, Phillips I M, Vijh S, Pamer E G. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity. 1998;8:353–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

