Cocirculation of avian H9N2 and contemporary "human" H3N2 influenza A viruses in pigs in southeastern China: potential for genetic reassortment?
- PMID: 11559800
- PMCID: PMC114539
- DOI: 10.1128/JVI.75.20.9679-9686.2001
Cocirculation of avian H9N2 and contemporary "human" H3N2 influenza A viruses in pigs in southeastern China: potential for genetic reassortment?
Abstract
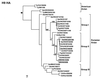
Pigs are permissive to both human and avian influenza viruses and have been proposed to be an intermediate host for the genesis of pandemic influenza viruses through reassortment or adaptation of avian viruses. Prospective virological surveillance carried out between March 1998 and June 2000 in Hong Kong, Special Administrative Region, People's Republic of China, on pigs imported from southeastern China, provides the first evidence of interspecies transmission of avian H9N2 viruses to pigs and documents their cocirculation with contemporary human H3N2 (A/Sydney/5/97-like, Sydney97-like) viruses. All gene segments of the porcine H9N2 viruses were closely related to viruses similar to chicken/Beijing/1/94 (H9N2), duck/Hong Kong/Y280/97 (H9N2), and the descendants of the latter virus lineage. Phylogenetic analysis suggested that repeated interspecies transmission events had occurred from the avian host to pigs. The Sydney97-like (H3N2) viruses isolated from pigs were related closely to contemporary human H3N2 viruses in all gene segments and had not undergone genetic reassortment. Cocirculation of avian H9N2 and human H3N2 viruses in pigs provides an opportunity for genetic reassortment leading to the emergence of viruses with pandemic potential.
Figures

References
-
- Bikour M H, Frost E H, Deslandes S, Talbot B, Weber J M, Elazhary Y. Recent H3N2 swine influenza virus with haemagglutinin and nucleoprotein genes similar to 1975 human strains. J Gen Virol. 1995;76:697–703. - PubMed
-
- Brown I H, Alexander D J, Chakraverty P, Harris P A, Manvell R J. Isolation of an influenza A virus of unusual subtype (H1N7) from pigs in England, and the subsequent experimental transmission from pig to pig. Vet Microbiol. 1994;39:125–134. - PubMed
-
- Brown I H, Harris P A, McCauley J W, Alexander D J. Multiple genetic reassortment of avian and human influenza A viruses in European pigs, resulting in the emergence of an H1N2 virus of novel genotype. J Gen Virol. 1998;79:2947–2955. - PubMed
-
- Cameron K R, Gregory V, Banks J, Brown I H, Alexander D J, Hay A J, Lin Y P. H9N2 subtype influenza A viruses in poultry in Pakistan are closely related to the H9N2 viruses responsible for human infection in Hong Kong. Virology. 2000;278:36–41. - PubMed
-
- Castrucci M R, Donatelli I, Sidoli L, Barigazzi G, Kawaoka Y, Webster R G. Genetic reassortment between avian and human influenza A viruses in Italian pigs. Virology. 1993;193:503–506. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

