In situ spatial organization of Potato virus A coat protein subunits as assessed by tritium bombardment
- PMID: 11559802
- PMCID: PMC114541
- DOI: 10.1128/JVI.75.20.9696-9702.2001
In situ spatial organization of Potato virus A coat protein subunits as assessed by tritium bombardment
Abstract
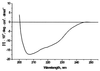
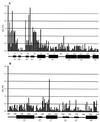
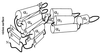
Potato virus A (PVA) particles were bombarded with thermally activated tritium atoms, and the intramolecular distribution of the label in the amino acids of the coat protein was determined to assess their in situ steric accessibility. This method revealed that the N-terminal 15 amino acids of the PVA coat protein and a region comprising amino acids 27 to 50 are the most accessible at the particle surface to labeling with tritium atoms. A model of the spatial arrangement of the PVA coat protein polypeptide chain within the virus particle was derived from the experimental data obtained by tritium bombardment combined with predictions of secondary-structure elements and the principles of packing alpha-helices and beta-structures in proteins. The model predicts three regions of tertiary structure: (i) the surface-exposed N-terminal region, comprising an unstructured N terminus of 8 amino acids and two beta-strands, (ii) a C-terminal region including two alpha-helices, as well as three beta-strands that form a two-layer structure called an abCd unit, and (iii) a central region comprising a bundle of four alpha-helices in a fold similar to that found in tobacco mosaic virus coat protein. This is the first model of the three-dimensional structure of a potyvirus coat protein.
Figures


Similar articles
-
The organization of potato virus X coat proteins in virus particles studied by tritium planigraphy and model building.Virology. 1992 May;188(1):175-80. doi: 10.1016/0042-6822(92)90747-d. Virology. 1992. PMID: 1566571
-
The topography of the surface of potato virus X: tritium planigraphy and immunological analysis.J Gen Virol. 1992 Feb;73 ( Pt 2):229-35. doi: 10.1099/0022-1317-73-2-229. J Gen Virol. 1992. PMID: 1371539
-
Changes in the Structure of Potato Virus A Virions after Limited in situ Proteolysis According to Tritium Labeling Data and Computer Simulation.Biochemistry (Mosc). 2023 Dec;88(12):2146-2156. doi: 10.1134/S0006297923120167. Biochemistry (Mosc). 2023. PMID: 38462457
-
Heating-induced transition of Potyvirus Potato Virus A coat protein into β-structure.J Biomol Struct Dyn. 2016;34(2):250-8. doi: 10.1080/07391102.2015.1022604. Epub 2015 Apr 8. J Biomol Struct Dyn. 2016. PMID: 25851284
-
[Investigation of helical plant virus ribonucleoprotein structures with the help of tritium planigraphy and theoretical modeling].Mol Biol (Mosk). 2004 Sep-Oct;38(5):945-58. Mol Biol (Mosk). 2004. PMID: 15554196 Review. Russian.
Cited by
-
Structure of flexible filamentous plant viruses.J Virol. 2008 Oct;82(19):9546-54. doi: 10.1128/JVI.00895-08. Epub 2008 Jul 30. J Virol. 2008. PMID: 18667514 Free PMC article.
-
Mutational analysis of interaction between coat protein and helper component-proteinase of Soybean mosaic virus involved in aphid transmission.Mol Plant Pathol. 2010 Mar;11(2):265-76. doi: 10.1111/j.1364-3703.2009.00603.x. Mol Plant Pathol. 2010. PMID: 20447275 Free PMC article.
-
Surface characterization of the thermal remodeling helical plant virus.PLoS One. 2019 May 31;14(5):e0216905. doi: 10.1371/journal.pone.0216905. eCollection 2019. PLoS One. 2019. PMID: 31150411 Free PMC article.
-
Analysis of the role of the coat protein N-terminal segment in Potato virus X virion stability and functional activity.Mol Plant Pathol. 2012 Jan;13(1):38-45. doi: 10.1111/j.1364-3703.2011.00725.x. Epub 2011 Jun 1. Mol Plant Pathol. 2012. PMID: 21726392 Free PMC article.
-
Analysis of potato virus Y coat protein epitopes recognized by three commercial monoclonal antibodies.PLoS One. 2014 Dec 26;9(12):e115766. doi: 10.1371/journal.pone.0115766. eCollection 2014. PLoS One. 2014. PMID: 25542005 Free PMC article.
References
-
- Andrejeva J, Järvekülg L, Rabenstein F, Torrance L, Harrison B D, Saarma M. Antigenic analysis of potato virus A particles and coat protein. Ann Appl Biol. 1994;125:337–348.
-
- Andrejeva J, Puurand Ü, Merits A, Rabenstein F, Järvekülg L, Valkonen J. Potyvirus helper component-proteinase and coat protein (CP) have co-ordinated functions in virus-host interactions and the same CP motif affects virus transmission and accumulation. J Gen Virol. 1999;80:1133–1139. - PubMed
-
- Atreya C D, Raccah B, Pirone T P. A point mutation in the coat protein abolishes aphid transmission of a potyvirus. Virology. 1990;178:161–165. - PubMed
-
- Atreya P L, Lopez-Moya J J, Chu M, Atreya C D, Pirone T P. Mutational analysis of the coat protein N-terminal amino acids involved in potyvirus transmission by aphids. J Gen Virol. 1995;76:265–270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

