Efficient virus extinction by combinations of a mutagen and antiviral inhibitors
- PMID: 11559805
- PMCID: PMC114544
- DOI: 10.1128/JVI.75.20.9723-9730.2001
Efficient virus extinction by combinations of a mutagen and antiviral inhibitors
Abstract
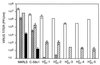
The effect of combinations of the mutagenic base analog 5-fluorouracil (FU) and the antiviral inhibitors guanidine hydrochloride (G) and heparin (H) on the infectivity of foot-and-mouth disease virus (FMDV) in cell culture has been investigated. Related FMDV clones differing up to 10(6)-fold in relative fitness in BHK-21 cells have been compared. Systematic extinction of intermediate fitness virus was attained with a combination of FU and G but not with the mutagen or the inhibitor alone. Systematic extinction of high-fitness FMDV required the combination of FU, G, and H. FMDV showing high relative fitness in BHK-21 cells but decreased replicative ability in CHO cells behaved as a low-fitness virus with regard to extinction mutagenesis in CHO cells. This confirms that relative fitness, rather than a specific genomic sequence, determines the FMDV response to enhanced mutagenesis. Mutant spectrum analysis of several genomic regions from a preextinction population showed a statistically significant increase in the number of mutations compared with virus passaged in parallel in the absence of FU and inhibitors. Also, in a preextinction population the types of mutations that can be attributed to the mutagenic action of FU were significantly more frequent than other mutation types. The results suggest that combinations of mutagenic agents and antiviral inhibitors can effectively drive high-fitness virus into extinction.
Figures


References
-
- Arias A, Lazaro E, Escarmis C, Domingo E. Molecular intermediates of fitness gain of an RNA virus: characterization of a mutant spectrum by biological and molecular cloning. J Gen Virol. 2001;82:1049–1060. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

