Multiple effector functions mediated by human immunodeficiency virus-specific CD4(+) T-cell clones
- PMID: 11559810
- PMCID: PMC114549
- DOI: 10.1128/JVI.75.20.9771-9779.2001
Multiple effector functions mediated by human immunodeficiency virus-specific CD4(+) T-cell clones
Abstract
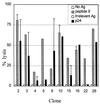
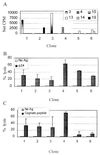
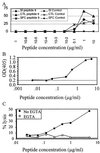
Mounting evidence suggests that human immunodeficiency virus type 1 (HIV-1) Gag-specific T helper cells contribute to effective antiviral control, but their functional characteristics and the precise epitopes targeted by this response remain to be defined. In this study, we generated CD4(+) T-cell clones specific for Gag from HIV-1-infected persons with vigorous Gag-specific responses detectable in peripheral blood mononuclear cells. Multiple peptides containing T helper epitopes were identified, including a minimal peptide, VHAGPIAG (amino acids 218 to 226), in the cyclophilin binding domain of Gag. Peptide recognition by all clones examined induced cell proliferation, gamma interferon (IFN-gamma) secretion, and cytolytic activity. Cytolysis was abrogated by concanamycin A and EGTA but not brefeldin A or anti-Fas antibody, implying a perforin-mediated mechanism of cell lysis. Additionally, serine esterase release into the extracellular medium, a marker for cytolytic granules, was demonstrated in an antigen-specific, dose-dependent fashion. These data indicate that T helper cells can target multiple regions of the p24 Gag protein and suggest that cytolytic activity may be a component of the antiviral effect of these cells.
Figures


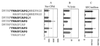

Similar articles
-
Fine specificity and cross-clade reactivity of HIV type 1 Gag-specific CD4+ T cells.AIDS Res Hum Retroviruses. 2004 Mar;20(3):315-25. doi: 10.1089/088922204322996554. AIDS Res Hum Retroviruses. 2004. PMID: 15117455 Free PMC article.
-
Diverse repertoire of HIV-1 p24-specific, IFN-gamma-producing CD4+ T cell clones following immune reconstitution on highly active antiretroviral therapy.J Immunol. 2003 Jan 15;170(2):1106-16. doi: 10.4049/jimmunol.170.2.1106. J Immunol. 2003. PMID: 12517980
-
Role of human immunodeficiency virus (HIV)-specific T-cell immunity in control of dual HIV-1 and HIV-2 infection.J Virol. 2007 Sep;81(17):9061-71. doi: 10.1128/JVI.00117-07. Epub 2007 Jun 20. J Virol. 2007. PMID: 17582003 Free PMC article.
-
CD4 responses to conserved HIV-1 T helper epitopes show both negative and positive associations with virus load in chronically infected subjects.Clin Exp Immunol. 2003 Dec;134(3):454-63. doi: 10.1111/j.1365-2249.2003.02307.x. Clin Exp Immunol. 2003. PMID: 14632751 Free PMC article.
-
Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity.J Immunol. 1996 May 15;156(10):3678-86. J Immunol. 1996. PMID: 8621902
Cited by
-
HIV-specific cytolytic CD4 T cell responses during acute HIV infection predict disease outcome.Sci Transl Med. 2012 Feb 29;4(123):123ra25. doi: 10.1126/scitranslmed.3003165. Sci Transl Med. 2012. PMID: 22378925 Free PMC article.
-
Cytotoxic HIV-1 p55gag-specific CD4+ T cells produce HIV-inhibitory cytokines and chemokines.J Clin Immunol. 2002 Sep;22(5):253-62. doi: 10.1023/a:1020066404226. J Clin Immunol. 2002. PMID: 12405158
-
Novel Nested Peptide Epitopes Recognized by CD4+ T Cells Induced by HIV-1 Conserved-Region Vaccines.Vaccines (Basel). 2020 Jan 16;8(1):28. doi: 10.3390/vaccines8010028. Vaccines (Basel). 2020. PMID: 31963212 Free PMC article.
-
Gag- and Nef-specific CD4+ T cells recognize and inhibit SIV replication in infected macrophages early after infection.Proc Natl Acad Sci U S A. 2009 Jun 16;106(24):9791-6. doi: 10.1073/pnas.0813106106. Epub 2009 May 28. Proc Natl Acad Sci U S A. 2009. PMID: 19478057 Free PMC article.
-
CD4+ T cells support production of simian immunodeficiency virus Env antibodies that enforce CD4-dependent entry and shape tropism in vivo.J Virol. 2013 Sep;87(17):9719-32. doi: 10.1128/JVI.01254-13. Epub 2013 Jul 3. J Virol. 2013. PMID: 23824793 Free PMC article.
References
-
- Alric L, Fort M, Izopet J, Vinel J P, Charlet J P, Selves J, Puel J, Pascal J P, Duffaut M, Abbal M. Genes of the major histocompatibility complex class II influence the outcome of hepatitis C virus infection. Gastroenterology. 1997;113:1675–1681. - PubMed
-
- Amara R R, Villinger F, Altman J D, Lydy S L, O'Neil S P, Staprans S I, Montefiori D C, Xu Y, Herndon J G, Wyatt L S, Candido M A, Kozyr N L, Earl P L, Smith J M, Ma H L, Grimm B D, Hulsey M L, Miller J, McClure H M, McNicholl J M, Moss B, Robinson H L. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292:69–74. - PubMed
-
- Barnaba V, Franco A, Paroli M, Benvenuto R, De Petrillo G, Burgio V L, Santilio I, Balsano C, Bonavita M S, Cappelli G. Selective expansion of cytotoxic T lymphocytes with a CD4+CD56+ surface phenotype and a T helper type 1 profile of cytokine secretion in the liver of patients chronically infected with Hepatitis B virus. J Immunol. 1994;152:3074–3087. - PubMed
-
- Barouch D, Santra S, Schmitz J, Kuroda M, Fu T, Wagner W, Bilska M, Craiu A, Zheng X, Krivulka G, Beaudry K, Lifton M, Nickerson C, Trigona W, Punt K, Freed D, Guan L, Dubey S, Casimiro D, Simon A, Davies M, Chastain M, Strom T, Gelman R, Montefiori D, Lewis M, Emini E, Shiver J, Letvin N. Control of viremia and prevention of clinical AIDS in Rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

