Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis
- PMID: 11559816
- PMCID: PMC114555
- DOI: 10.1128/JVI.75.20.9828-9835.2001
Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis
Abstract
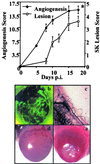
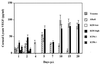
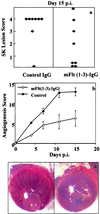
This report analyzes the role of vascular endothelial growth factor (VEGF)-induced angiogenesis in the immunoinflammatory lesion stromal keratitis induced by ocular infection with herpes simplex virus (HSV). Our results show that infection with replication-competent, but not mutant, viruses results in the expression of VEGF mRNA and protein in the cornea. This a rapid event, with VEGF mRNA detectable by 12 h postinfection (p.i.) and proteins detectable by 24 h p.i. VEGF production occurred both in the virus-infected corneal epithelium and in the underlying stroma, in which viral antigens were undetectable. In the stroma, VEGF was produced by inflammatory cells; these initially were predominantly polymorphonuclear leukocytes (PMN), but at later time points both PMN and macrophage-like cells were VEGF producers. In the epithelium, the major site of VEGF-expressing cells in early infection, the infected cells themselves were usually negative for VEGF. Similarly, in vitro infection studies indicated that the cells which produced VEGF were not those which expressed virus. Attesting to the possible role of VEGF-induced angiogenesis in the pathogenesis of herpetic stromal keratitis were experiments showing that VEGF inhibition with mFlt(1-3)-immunoglobulin G diminished angiogenesis and the severity of lesions after HSV infection. These observations are the first to evaluate VEGF-induced angiogenesis in the pathogenesis of stromal keratitis. Our results indicate that the control of angiogenesis represents a useful adjunct to therapy of herpetic ocular disease, an important cause of human blindness.
Figures




Similar articles
-
Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes: therapeutic strategy for herpetic stromal keratitis.Am J Pathol. 2004 Dec;165(6):2177-85. doi: 10.1016/S0002-9440(10)63267-1. Am J Pathol. 2004. PMID: 15579459 Free PMC article.
-
Control of stromal keratitis by inhibition of neovascularization.Am J Pathol. 2001 Sep;159(3):1021-9. doi: 10.1016/S0002-9440(10)61777-4. Am J Pathol. 2001. PMID: 11549594 Free PMC article.
-
Role of inflammatory cytokine-induced cyclooxygenase 2 in the ocular immunopathologic disease herpetic stromal keratitis.J Virol. 2005 Aug;79(16):10589-600. doi: 10.1128/JVI.79.16.10589-10600.2005. J Virol. 2005. PMID: 16051851 Free PMC article.
-
Corneal lymphangiogenesis in herpetic stromal keratitis.Surv Ophthalmol. 2015 Jan-Feb;60(1):60-71. doi: 10.1016/j.survophthal.2014.06.001. Epub 2014 Jun 10. Surv Ophthalmol. 2015. PMID: 25444520 Free PMC article. Review.
-
Herpes simplex virus keratitis in children.Am J Ophthalmol. 2004 Sep;138(3):474-5. doi: 10.1016/j.ajo.2004.04.027. Am J Ophthalmol. 2004. PMID: 15364233 Review.
Cited by
-
Role of matrix metalloproteinase-9 in angiogenesis caused by ocular infection with herpes simplex virus.J Clin Invest. 2002 Oct;110(8):1105-11. doi: 10.1172/JCI15755. J Clin Invest. 2002. PMID: 12393846 Free PMC article.
-
An anti-inflammatory role of VEGFR2/Src kinase inhibitor in herpes simplex virus 1-induced immunopathology.J Virol. 2011 Jun;85(12):5995-6007. doi: 10.1128/JVI.00034-11. Epub 2011 Apr 6. J Virol. 2011. PMID: 21471229 Free PMC article.
-
Activated inflammatory infiltrate in HSV-1-infected corneas without herpes stromal keratitis.Invest Ophthalmol Vis Sci. 2008 Apr;49(4):1488-95. doi: 10.1167/iovs.07-1107. Invest Ophthalmol Vis Sci. 2008. PMID: 18385067 Free PMC article.
-
Absence of CXCL10 aggravates herpes stromal keratitis with reduced primary neutrophil influx in mice.J Virol. 2013 Aug;87(15):8502-10. doi: 10.1128/JVI.01198-13. Epub 2013 May 29. J Virol. 2013. PMID: 23720717 Free PMC article.
-
Suppression of transcription factor early growth response 1 reduces herpes simplex virus 1-induced corneal disease in mice.J Virol. 2012 Aug;86(16):8559-67. doi: 10.1128/JVI.00505-12. Epub 2012 May 30. J Virol. 2012. PMID: 22647700 Free PMC article.
References
-
- Aiello L P, Pierce E A, Foley E D, Takagi H, Chen H, Riddle L, Ferrara N, King G L, Smith L E. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA. 1995;92:10457–10461. - PMC - PubMed
-
- Aoki Y, Yarchoan R, Wyvill K, Okamoto S, Little R F, Tosato G. Detection of viral interleukin-6 in Kaposi sarcoma-associated herpesvirus-linked disorders. Blood. 2001;97:2173–2176. - PubMed
-
- Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka E G, Gutkind J S, Asch A S, Cesarman E, Gershengorn M C, Mesri E A, Gerhengorn M C. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a v oncogene and angiogenesis activator. Nature. 1998;391:86–89. - PubMed
-
- Boshoff C. Kaposi's sarcoma. Coupling herpesvirus to angiogenesis. Nature. 1998;391:24–25. - PubMed
-
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

