Transient disruption of intercellular junctions enables baculovirus entry into nondividing hepatocytes
- PMID: 11559819
- PMCID: PMC114558
- DOI: 10.1128/JVI.75.20.9857-9871.2001
Transient disruption of intercellular junctions enables baculovirus entry into nondividing hepatocytes
Abstract
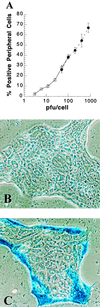
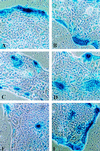
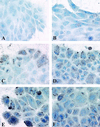
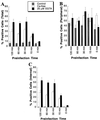
Baculovirus infection has extended the capabilities for transfection of exogenous genes into a variety of mammalian cell types. Because rat hepatocytes plated on collagen-coated dishes and maintained in dimethyl sulfoxide (DMSO)-supplemented chemically defined medium are an excellent model system for studying liver function in vitro, we investigated the ability of baculoviruses to infect and deliver exogenous genes to cells in this culture system. Efficient delivery to hepatocytes in short-term culture becomes restricted to peripheral cells, or "edge" cells, as the hepatocytes acquire intercellular junctions and form islands with time in culture. This barrier to baculovirus entry can be overcome, and the percentage of internal cells within the hepatocyte islands that are infected with the baculovirus can be increased more than 100-fold, when cells are subjected to transient calcium depletion before and during infection. These findings suggest that at least in some cell types, such as hepatocytes, baculovirus entry may require contact with the basolateral surface. We conclude from this study that recombinant baculovirus infection following transient depletion of extracellular calcium results in delivery of exogenous genes to at least 75% of hepatocytes in long-term DMSO culture, thereby making it possible for the first time to carry out gain-of-function and loss-of-function studies in this cell system.
Figures





References
-
- Bhat M, Toledo-Velasquez D, Wang L, Malanga C J, Ma J K H, Rojanasakul Y. Regulation of tight junction permeability by calcium mediators and cell cytoskeleton in rabbit tracheal epithelium. Pharm Res. 1993;10:991–997. - PubMed
-
- Borrmann C M, Mertens C, Schmidt A, Langbein L, Kuhn C, Franke W W. Molecular diversity of plaques of epithelial-adhering junctions. Ann N Y Acad Sci. 2000;915:144–150. - PubMed
-
- Boyce F M, Franco E A. High-efficiency transduction of mammalian cells using baculovirus: practical aspects. In: Cid-Arrequi A, Garcia-Carranca A, editors. Viral vectors: basic science and gene therapy. Natick, Mass: Eaton Publishing; 2000. pp. 359–367.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

