Saquinavir and ritonavir pharmacokinetics following combined ritonavir and saquinavir (soft gelatin capsules) administration
- PMID: 11560557
- PMCID: PMC2014551
- DOI: 10.1046/j.0306-5251.2001.01452.x
Saquinavir and ritonavir pharmacokinetics following combined ritonavir and saquinavir (soft gelatin capsules) administration
Abstract
Aims: To investigate the influence of combined ritonavir (RTV) and saquinavir (soft-gelatin capsule formulation; SQV) on systemic exposure to SQV with a view to optimizing the dosing regimen of combined RTV and SQV antiretroviral therapy.
Methods: In this open labelled, randomized, parallel group study, SQV and RTV were administered twice daily for 14 days to groups of eight healthy subjects. The two antiretrovirals were either administered alone (800 mg SQV, regimen A, and 400 mg RTV, B) or in combination at various dose levels (RTV : SQV: 400 : 400 mg, C; 300 : 600 mg, D; 200 : 800 mg, E; 300 : 800 mg, F; 400 : 800 mg, G; and 400 : 600 mg, H). Pharmacokinetic parameters of saquinavir and ritonavir were determined and adverse events, vital signs, and clinical laboratory variables recorded.
Results: RTV substantially increased the plasma concentration of saquinavir for all dose combinations, compared with SQV alone. Based on the primary statistical analysis there was an overall 17-, 22-, and 23-fold increase in saquinavir AUC(0,24 h) on day 14 with regimens E, F, and G, respectively (with confidence intervals of 10-30, 13-37, and 13-39). The lowest combination dose of RTV (200 : 800 mg; E) significantly increased the saquinavir AUC(0,24 h) from below 5 to 57 microg ml(-1) h, which was higher than the exposure obtained with the 400 : 400 mg twice daily regimen (i.e. 36 microg ml(-1) h). RTV also reduced intersubject variability in AUC(0,24 h) for saquinavir from 105% to 32-68%, and C(max)(0,24 h) from 124% to 30-49%. In contrast, SQV showed no clinically significant effect on the pharmacokinetics of ritonavir. The combination regimens were well tolerated, with the least number of adverse events recorded for the 200 : 800 mg (RTV : SQV) combination regimen.
Conclusions: RTV significantly increases saquinavir exposure as a consequence of inhibiting SQV metabolism and possibly P-glycoprotein efflux. Pharmacokinetic and safety profiles obtained in the current study indicate that the use of a combination with a lower dose of RTV and a higher dose of SQV than the 400 : 400 mg combination frequently used in clinical practice should be further explored.
Figures
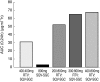
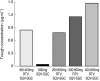
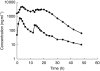
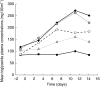
 300:800 mg (regimen F,
300:800 mg (regimen F,  ), or 400:800 mg (regimen G, ♦) combination RTV: SQV-SGC therapy twice daily.
), or 400:800 mg (regimen G, ♦) combination RTV: SQV-SGC therapy twice daily.  RTV 400 mg (regimen B).
RTV 400 mg (regimen B).Similar articles
-
Steady-state pharmacokinetics of twice-daily dosing of saquinavir plus ritonavir in HIV-1-infected individuals.J Acquir Immune Defic Syndr. 2001 Aug 1;27(4):344-9. doi: 10.1097/00126334-200108010-00004. J Acquir Immune Defic Syndr. 2001. PMID: 11468422 Clinical Trial.
-
Pharmacokinetics of once-daily saquinavir hard-gelatin capsules and saquinavir soft-gelatin capsules boosted with ritonavir in HIV-1-infected subjects.J Acquir Immune Defic Syndr. 2003 Apr 1;32(4):375-9. doi: 10.1097/00126334-200304010-00005. J Acquir Immune Defic Syndr. 2003. PMID: 12640194 Clinical Trial.
-
Pharmacokinetic and tolerability profile of twice-daily saquinavir hard gelatin capsules and saquinavir soft gelatin capsules boosted with ritonavir in healthy volunteers.HIV Med. 2003 Apr;4(2):94-100. doi: 10.1046/j.1468-1293.2003.00143.x. HIV Med. 2003. PMID: 12702129 Clinical Trial.
-
Saquinavir soft-gel capsule: an updated review of its use in the management of HIV infection.Drugs. 2000 Aug;60(2):481-516. doi: 10.2165/00003495-200060020-00016. Drugs. 2000. PMID: 10983742 Review.
-
Amprenavir or fosamprenavir plus ritonavir in HIV infection: pharmacology, efficacy and tolerability profile.Drugs. 2005;65(5):633-59. doi: 10.2165/00003495-200565050-00005. Drugs. 2005. PMID: 15748098 Review.
Cited by
-
Potencies of human immunodeficiency virus protease inhibitors in vitro against Plasmodium falciparum and in vivo against murine malaria.Antimicrob Agents Chemother. 2006 Feb;50(2):639-48. doi: 10.1128/AAC.50.2.639-648.2006. Antimicrob Agents Chemother. 2006. PMID: 16436721 Free PMC article.
-
Adherence to antiretroviral therapy and its impact on clinical outcome in HIV-infected patients.J R Soc Interface. 2005 Sep 22;2(4):349-63. doi: 10.1098/rsif.2005.0037. J R Soc Interface. 2005. PMID: 16849193 Free PMC article.
-
Saquinavir: a review of its use in boosted regimens for treating HIV infection.Drugs. 2003;63(12):1299-324. doi: 10.2165/00003495-200363120-00007. Drugs. 2003. PMID: 12790697 Review.
-
The effects of ritonavir and lopinavir/ritonavir on the pharmacokinetics of a novel CCR5 antagonist, aplaviroc, in healthy subjects.Br J Clin Pharmacol. 2006 Sep;62(3):336-44. doi: 10.1111/j.1365-2125.2006.02661.x. Br J Clin Pharmacol. 2006. PMID: 16934050 Free PMC article.
-
Membrane Assays to Characterize Interaction of Drugs with ABCB1.J Membr Biol. 2015 Dec;248(6):967-77. doi: 10.1007/s00232-015-9804-y. Epub 2015 Apr 30. J Membr Biol. 2015. PMID: 25926125
References
-
- Stellbrink H-J. Abstracts from the Sixth European Conference on Clinical Aspects and Treatment of HIV-Infection. Hamburg, Germany: 1997. Clinical and survival benefit of saquinavir (SQV) in combination with zalcitabine (DDC) and zidovudine (ZDV) in untreated minimally treated HIV-infected patients; p. 21. abstract 212.
-
- Haubrich R, Lalezari J, Follansbee SE, et al. Improved survival and reduced clinical progression in HIV-infected patients with advanced disease treated with saquinavir plus zalcitabine. Antiviral Ther. 1998;3:33–42.
-
- Cohen Stuart JWT, Schuurman R, Burger DM, et al. Randomized trial comparing saquinavir soft gelatin capsules versus indinavir as part of triple therapy (CHEESE study) AIDS. 1999;13:F53–F58. - PubMed
-
- Mitsuyasu RT, Skolnik PR, Cohen SR, et al. Activity of the soft gelatin formulation of saquinavir in combination therapy in antiretroviral-naive patients. AIDS. 1998;12:F103–F109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

