Plasma concentrations of haloperidol are related to CYP2D6 genotype at low, but not high doses of haloperidol in Korean schizophrenic patients
- PMID: 11560558
- PMCID: PMC2014539
- DOI: 10.1046/j.0306-5251.2001.01437.x
Plasma concentrations of haloperidol are related to CYP2D6 genotype at low, but not high doses of haloperidol in Korean schizophrenic patients
Abstract
Aims: This study was carried out to evaluate the influence of CYP2D6 genotype on the steady state plasma concentrations of haloperidol and reduced haloperidol in Korean schizophrenic patients.
Methods: One hundred and twenty Korean schizophrenic patients treated with various, clinically determined, doses of haloperidol (range 3-60, median 20 mg day-1) during monotherapy were recruited. CYP2D6 genotypes were determined by analysis of the CYP2D6*10 allele using allele-specific PCR and the CYP2D6*5 allele by long-PCR. Steady state plasma concentrations of haloperidol and reduced haloperidol were analysed by h.p.l.c.
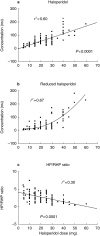
Results: Twenty-three (19.2%), 60 (50.0%), 1 (0.8%), 33 (27.5%) and 3 patients (2.5%) possessed the CYP2D6 genotypes *1/*1, *1/*10, *1/*5, *10/*10 and *10/*5, respectively. The allele frequencies of CYP2D6*1, *10 and *5 were 44.6%, 53.8% and 1.7%, respectively. Significant relationships between dose and plasma concentrations of haloperidol (linear; r2 = 0.60, P < 0.0001) and reduced haloperidol (quadratic equation; r(2) = 0.67) were observed. Overall, the concentrations normalized for dose (C/D) of haloperidol were significantly different between the CYP2D6*1/*1, *1/*10 and *10/*10 genotype groups (one-way ANOVA; P = 0.028). No significant differences between the genotype groups were found with respect to the C/D of reduced haloperidol (P = 0.755). However, in patients with daily doses less than 20 mg, significant differences in the C/D of haloperidol (P = 0.003), but not of reduced haloperidol, were found between the three major genotype groups. In patients with doses higher than 20 mg, no differences were found between the genotype groups for either haloperidol or reduced haloperidol. 68 patients (57%) used benztropine, an antimuscarinic agent. All four patients with a *5 allele (one together with *1 and three with *10) were found to use benztropine. The patients homozygous for the *1 allele seemed to need less benztropine than the patients with one or two mutated alleles (Fisher's exact test; P = 0.036).
Conclusions: The dose-corrected steady state plasma concentrations of haloperidol, but not of reduced haloperidol, were significantly different between the CYP2D6*1/*1, *1/*10 and *10/*10 genotype groups when doses lower than 20 mg haloperidol were given. No differences were found at higher doses. These results suggest the involvement of CYP2D6 in the metabolism of haloperidol at low doses of haloperidol (< 20 mg daily), while another enzyme, probably CYP3A4, contributes at higher doses.
Figures

Similar articles
-
The effect of cytochrome P450 2D6 genotypes on haloperidol metabolism: a preliminary study in a psychiatric population.Psychiatry Clin Neurosci. 1999 Oct;53(5):593-7. doi: 10.1046/j.1440-1819.1999.00611.x. Psychiatry Clin Neurosci. 1999. PMID: 10595685
-
Depot haloperidol treatment in outpatients with schizophrenia on monotherapy: impact of CYP2D6 polymorphism on pharmacokinetics and treatment outcome.Ther Drug Monit. 2007 Aug;29(4):417-22. doi: 10.1097/FTD.0b013e31811f394d. Ther Drug Monit. 2007. PMID: 17667795
-
Risperidone metabolism in relation to CYP2D6*10 allele in Korean schizophrenic patients.Eur J Clin Pharmacol. 2001 Nov;57(9):671-5. doi: 10.1007/s002280100372. Eur J Clin Pharmacol. 2001. PMID: 11791898
-
[Clinical pharmacogenetics in the treatment of schizophrenia].Nihon Shinkei Seishin Yakurigaku Zasshi. 2005 Jun;25(3):129-35. Nihon Shinkei Seishin Yakurigaku Zasshi. 2005. PMID: 16045195 Review. Japanese.
-
[Clinical pharmacogenetics in the treatment of schizophrenia].Nihon Shinkei Seishin Yakurigaku Zasshi. 2010 Apr;30(2):65-9. Nihon Shinkei Seishin Yakurigaku Zasshi. 2010. PMID: 20491279 Review. Japanese.
Cited by
-
Effect of CYP2D6*10 on pharmacokinetic variability of routinely administered metoprolol in middle-aged and elderly Japanese patients.Eur J Clin Pharmacol. 2003 Sep;59(5-6):385-8. doi: 10.1007/s00228-003-0638-7. Epub 2003 Aug 12. Eur J Clin Pharmacol. 2003. PMID: 12915955 Clinical Trial.
-
Molecular genetics of CYP2D6: clinical relevance with focus on psychotropic drugs.Br J Clin Pharmacol. 2002 Feb;53(2):111-22. doi: 10.1046/j.0306-5251.2001.01548.x. Br J Clin Pharmacol. 2002. PMID: 11851634 Free PMC article. Review.
-
Using in silico methods to determine optimal tapering regimens for decanoate-based long-acting injectable psychosis drugs.Ther Adv Psychopharmacol. 2024 Sep 12;14:20451253241272790. doi: 10.1177/20451253241272790. eCollection 2024. Ther Adv Psychopharmacol. 2024. PMID: 39282238 Free PMC article.
-
Effects of dosage and CYP2D6-mutated allele on plasma concentration of paroxetine.Eur J Clin Pharmacol. 2004 Oct;60(8):553-7. doi: 10.1007/s00228-004-0792-6. Epub 2004 Sep 3. Eur J Clin Pharmacol. 2004. PMID: 15349705
-
Ethnic or racial differences revisited: impact of dosage regimen and dosage form on pharmacokinetics and pharmacodynamics.Clin Pharmacokinet. 2006;45(10):957-64. doi: 10.2165/00003088-200645100-00001. Clin Pharmacokinet. 2006. PMID: 16984210 Review.
References
-
- Forsman A, Folsch G, Larsson M, Ohman R. On the metabolism of haloperidol in man. Curr Ther Res. 1977;21:606–617.
-
- Forsman A, Larsson M. Metabolism of haloperidol. Curr Ther Res. 1978;24:567–568.
-
- Pape BE. Isolation and identification of a metabolite of haloperidol. J Anal Toxicol. 1981;5:113–117. - PubMed
-
- Oida T, Terauchi Y, Yoshida K, Kagemoto A. Use of antisera in the isolation of human specific conjugates of haloperidol. Xenobiotica. 1989;19:781–793. - PubMed
-
- Eyles DW, McLennan HR, Jones A, McGrath JJ, Stedman TJ, Pond SM. Quantitative analysis of two pyridinium metaobolites of haloperidol in patients with schizophrenia. Clin Pharmacol Ther. 1994;56:512–520. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

