Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses
- PMID: 11560997
- PMCID: PMC2195955
- DOI: 10.1084/jem.194.6.823
Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses
Abstract
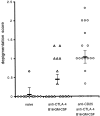
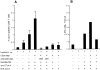
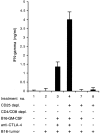
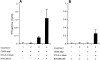
Therapeutic efficacy of a tumor cell-based vaccine against experimental B16 melanoma requires the disruption of either of two immunoregulatory mechanisms that control autoreactive T cell responses: the cytotoxic T lymphocyte-associated antigen (CTLA)-4 pathway or the CD25(+) regulatory T (Treg) cells. Combination of CTLA-4 blockade and depletion of CD25(+) Treg cells results in maximal tumor rejection. Efficacy of the antitumor therapy correlates with the extent of autoimmune skin depigmentation as well as with the frequency of tyrosinase-related protein 2(180-188)-specific CTLs detected in the periphery. Furthermore, tumor rejection is dependent on the CD8(+) T cell subset. Our data demonstrate that the CTL response against melanoma antigens is an important component of the therapeutic antitumor response and that the reactivity of these CTLs can be augmented through interference with immunoregulatory mechanisms. The synergism in the effects of CTLA-4 blockade and depletion of CD25(+) Treg cells indicates that CD25(+) Treg cells and CTLA-4 signaling represent two alternative pathways for suppression of autoreactive T cell immunity. Simultaneous intervention with both regulatory mechanisms is therefore a promising concept for the induction of therapeutic antitumor immunity.
Figures




Similar articles
-
Generation of antitumor immunity by cytotoxic T lymphocyte epitope peptide vaccination, CpG-oligodeoxynucleotide adjuvant, and CTLA-4 blockade.Cancer Res. 2003 Jun 15;63(12):3281-8. Cancer Res. 2003. PMID: 12810660
-
Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation.J Exp Med. 1999 Aug 2;190(3):355-66. doi: 10.1084/jem.190.3.355. J Exp Med. 1999. PMID: 10430624 Free PMC article.
-
Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy.J Exp Med. 2001 Aug 20;194(4):481-9. doi: 10.1084/jem.194.4.481. J Exp Med. 2001. PMID: 11514604 Free PMC article.
-
CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy.Annu Rev Immunol. 2001;19:565-94. doi: 10.1146/annurev.immunol.19.1.565. Annu Rev Immunol. 2001. PMID: 11244047 Review.
-
Manipulation of regulatory T cells and antigen-specific cytotoxic T lymphocyte-based tumour immunotherapy.Immunology. 2015 Feb;144(2):186-96. doi: 10.1111/imm.12387. Immunology. 2015. PMID: 25243729 Free PMC article. Review.
Cited by
-
Highly clonal regulatory T-cell population in follicular lymphoma - inverse correlation with the diversity of CD8+ T cells.Oncoimmunology. 2015 Feb 3;4(5):e1002728. doi: 10.1080/2162402X.2014.1002728. eCollection 2015 May. Oncoimmunology. 2015. PMID: 26155390 Free PMC article.
-
Regulation of COX2 expression in mouse mammary tumor cells controls bone metastasis and PGE2-induction of regulatory T cell migration.PLoS One. 2012;7(9):e46342. doi: 10.1371/journal.pone.0046342. Epub 2012 Sep 28. PLoS One. 2012. PMID: 23029485 Free PMC article.
-
Increase of CD4+ CD25+ regulatory T cells in the peripheral blood of patients with metastatic carcinoma: a Phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4+ CD25+ T lymphocytes.Clin Exp Immunol. 2007 Dec;150(3):523-30. doi: 10.1111/j.1365-2249.2007.03521.x. Epub 2007 Oct 22. Clin Exp Immunol. 2007. PMID: 17956583 Free PMC article. Clinical Trial.
-
Regulatory T cells and tumor immunity.Cancer Immunol Immunother. 2005 Dec;54(12):1153-61. doi: 10.1007/s00262-005-0699-9. Epub 2005 May 3. Cancer Immunol Immunother. 2005. PMID: 15868167 Free PMC article. Review.
-
Regulation of tumour immunity by CD25+ T cells.Immunology. 2002 Sep;107(1):5-9. doi: 10.1046/j.1365-2567.2002.01471.x. Immunology. 2002. PMID: 12225357 Free PMC article. Review. No abstract available.
References
-
- Melief C.J., Toes R.E., Medema J.P., van der Burg S.H., Ossendorp F., Offringa R. Strategies for immunotherapy of cancer. Adv. Immunol. 2000;75:235–282. - PubMed
-
- Kawakami Y., Rosenberg S.A. T-cell recognition of self peptides as tumor rejection antigens. Immunol. Res. 1996;15:179–190. - PubMed
-
- Allison J.P. CD28-B7 interactions in T-cell activation. Curr. Opin. Immunol. 1994;6:414–419. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

