Structure of rat BCKD kinase: nucleotide-induced domain communication in a mitochondrial protein kinase
- PMID: 11562470
- PMCID: PMC58710
- DOI: 10.1073/pnas.201220098
Structure of rat BCKD kinase: nucleotide-induced domain communication in a mitochondrial protein kinase
Abstract
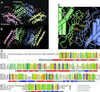
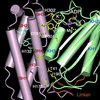
Mitochondrial protein kinases (mPKs) are molecular switches that down-regulate the oxidation of branched-chain alpha-ketoacids and pyruvate. Elevated levels of these metabolites are implicated in disease states such as insulin-resistant Type II diabetes, branched-chain ketoaciduria, and primary lactic acidosis. We report a three-dimensional structure of a member of the mPK family, rat branched-chain alpha-ketoacid dehydrogenase kinase (BCK). BCK features a characteristic nucleotide-binding domain and a four-helix bundle domain. These two domains are reminiscent of modules found in protein histidine kinases (PHKs), which are involved in two-component signal transduction systems. Unlike PHKs, BCK dimerizes through direct interaction of two opposing nucleotide-binding domains. Nucleotide binding to BCK is uniquely mediated by both potassium and magnesium. Binding of ATP induces disorder-order transitions in a loop region at the nucleotide-binding site. These structural changes lead to the formation of a quadruple aromatic stack in the interface between the nucleotide-binding domain and the four-helix bundle domain, where they induce a movement of the top portion of two helices. Phosphotransfer induces further ordering of the loop region, effectively trapping the reaction product ADP, which explains product inhibition in mPKs. The BCK structure is a prototype for all mPKs and will provide a framework for structure-assisted inhibitor design for this family of kinases.
Figures

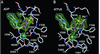

References
-
- Chuang D T, Shih V E. In: The Metabolic and Molecular Basis of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 2001. pp. 1971–2006.
-
- Chuang D T, Hu C W, Ku L S, Markovitz P J, Cox R P. J Biol Chem. 1985;260:13779–13786. - PubMed
-
- Paxton R, Harris R A. J Biol Chem. 1982;257:14433–14439. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

