HS-599: a novel long acting opioid analgesic does not induce place-preference in rats
- PMID: 11564664
- PMCID: PMC1572965
- DOI: 10.1038/sj.bjp.0704280
HS-599: a novel long acting opioid analgesic does not induce place-preference in rats
Abstract
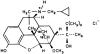
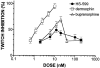
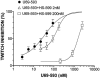
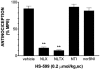
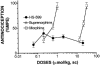
1. When administered subcutaneously HS-599, a new didehydroderivative of buprenorphine (18,19-dehydrobuprenorphine), produced a long-lasting antinociceptive response in rats. Its potency exceeded twice that of buprenorphine. In the tail-flick test it acted as a full agonist but in the plantar test only as a partial agonist. Whereas the mu-opioid antagonists naloxone and naltrexone antagonized HS-599 antinociception the delta-opioid antagonist naltrindole and the kappa-opioid antagonist nor-binaltorphimine did not. 2. Unlike buprenorphine and morphine, HS-599 never induced conditioned place-preference in rats. 3. In radioligand binding assays, compared with buprenorphine HS-599 had 3 fold higher mu-opioid receptor affinity but lower delta- and kappa-opioid receptor affinity. 4. In isolated guinea-pig ileum preparations, HS-599 only partially inhibited the electrically-stimulated contraction, acting as a partial opioid agonist. When tested against the mu-opioid receptor agonist dermorphin, it behaved as a non-equilibrium antagonist. Conversely, in mouse vas deferens (rich in delta-opioid receptors) and rabbit vas deferens preparations (rich in kappa-opioid receptors) HS-599 acted as a pure equilibrium antagonist, shifting the log-concentration-response curves of the delta-opioid agonist deltorphin I and the kappa-opioid agonist U-69593 to the right. 5. In conclusion, HS-599 is a novel buprenorphine derivative with higher affinity, selectivity and potency than the parent compound, for mu-opioid receptors. It produces intense and long-lasting antinociception and does not induce place-preference in rats.
Figures


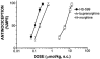

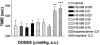
Similar articles
-
Antinociceptive activity of a novel buprenorphine analogue.Life Sci. 2002 Mar 22;70(18):2177-85. doi: 10.1016/s0024-3205(01)01553-3. Life Sci. 2002. PMID: 12002809
-
Characterization of the complex morphinan derivative BU72 as a high efficacy, long-lasting mu-opioid receptor agonist.Eur J Pharmacol. 2004 Sep 19;499(1-2):107-16. doi: 10.1016/j.ejphar.2004.07.097. Eur J Pharmacol. 2004. PMID: 15363957
-
Some pharmacological properties of a newly synthesized 3-acetoxy-6 beta-acetylthio-10-oxo-N-cyclopropylmethyl-dihydronormorphine (KT-95).Arch Int Pharmacodyn Ther. 1996 Mar-Apr;331(2):136-52. Arch Int Pharmacodyn Ther. 1996. PMID: 8937625
-
Buprenorphine: an analgesic with an expanding role in the treatment of opioid addiction.CNS Drug Rev. 2002 Winter;8(4):377-90. doi: 10.1111/j.1527-3458.2002.tb00235.x. CNS Drug Rev. 2002. PMID: 12481193 Free PMC article. Review.
-
Buprenorphine.Drug Alcohol Depend. 1985 Feb;14(3-4):363-72. doi: 10.1016/0376-8716(85)90067-5. Drug Alcohol Depend. 1985. PMID: 2986930 Review.
Cited by
-
TH-030418: a potent long-acting opioid analgesic with low dependence liability.Naunyn Schmiedebergs Arch Pharmacol. 2011 Aug;384(2):125-31. doi: 10.1007/s00210-011-0652-8. Epub 2011 May 19. Naunyn Schmiedebergs Arch Pharmacol. 2011. PMID: 21594658
-
Buprenorphine reduces alcohol drinking through activation of the nociceptin/orphanin FQ-NOP receptor system.Biol Psychiatry. 2007 Jan 1;61(1):4-12. doi: 10.1016/j.biopsych.2006.01.006. Epub 2006 Mar 14. Biol Psychiatry. 2007. PMID: 16533497 Free PMC article.
-
The first universal opioid ligand, (2S)-2-[(5R,6R,7R,14S)-N-cyclopropylmethyl-4,5-epoxy-6,14-ethano-3-hydroxy-6-methoxymorphinan-7-yl]-3,3-dimethylpentan-2-ol (BU08028): characterization of the in vitro profile and in vivo behavioral effects in mouse models of acute pain and cocaine-induced reward.J Pharmacol Exp Ther. 2011 Mar;336(3):952-61. doi: 10.1124/jpet.110.175620. Epub 2010 Dec 21. J Pharmacol Exp Ther. 2011. PMID: 21177476 Free PMC article.
-
Reassessment of buprenorphine in conditioned place preference: temporal and pharmacological considerations.Psychopharmacology (Berl). 2004 Feb;172(1):58-67. doi: 10.1007/s00213-003-1626-4. Epub 2003 Nov 13. Psychopharmacology (Berl). 2004. PMID: 14615874
References
-
- BICKEL W.K., STITZER M.L., BIGELOW G.E., LIEBSON I.A., JANINSKI D.R., JOHNSON R.E. A clinical trial of buprenorphine: comparison with methadone in the detoxification of heroin addicts. Clin. Pharmacol. Ther. 1988;43:72–78. - PubMed
-
- BROWN E.E., FINLAY J.M., WONG J.T.F., DAMSMA G., FIBIGER H.C. Behavioral and neurochemical interactions between cocaine and buprenorphine: implications for the pharmacotherapy of cocaine abuse. J. Pharmacol. Exp. Ther. 1991;256:119–126. - PubMed
-
- CHOWDHURY A.N., CHOWDHURY S. Buprenorphine abuse: report from India. Br. J. Addict. 1990;85:1349–1350. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

