Vps34p differentially regulates endocytosis from the apical and basolateral domains in polarized hepatic cells
- PMID: 11564757
- PMCID: PMC2150819
- DOI: 10.1083/jcb.200105138
Vps34p differentially regulates endocytosis from the apical and basolateral domains in polarized hepatic cells
Abstract
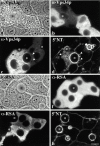
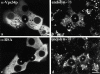
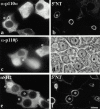
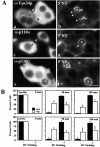
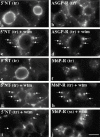
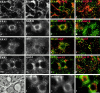
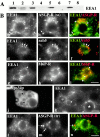
Using a microinjection approach to study apical plasma membrane protein trafficking in hepatic cells, we found that specific inhibition of Vps34p, a class III phosphoinositide 3 (PI-3) kinase, nearly perfectly recapitulated the defects we reported for wortmannin-treated cells (Tuma, P.L., C.M. Finnegan, J.-H Yi, and A.L. Hubbard. 1999. J. Cell Biol. 145:1089-1102). Both wortmannin and injection of inhibitory Vps34p antibodies led to the accumulation of resident apical proteins in enlarged prelysosomes, whereas transcytosing apical proteins and recycling basolateral receptors transiently accumulated in basolateral early endosomes. To understand how the Vps34p catalytic product, PI3P, was differentially regulating endocytosis from the two domains, we examined the PI3P binding protein early endosomal antigen 1 (EEA1). We determined that EEA1 distributed to two biochemically distinct endosomal populations: basolateral early endosomes and subapical endosomes. Both contained rab5, although the latter also contained late endosomal markers but was distinct from the transcytotic intermediate, the subapical compartment. When PI3P was depleted, EEA1 dissociated from basolateral endosomes, whereas it remained on subapical endosomes. From these results, we conclude that PI3P, via EEA1, regulates early steps in endocytosis from the basolateral surface in polarized WIF-B cells. However, PI3P must use different machinery in its regulation of the apical endocytic pathway, since later steps are affected by Vps34p inhibition.
Figures


References
-
- Backer, J.M. 2000. Phosphoinositide 3-kinases and the regulation of vesicular trafficking. Mol. Cell. Biol. Res. Commun. 3:193–204. - PubMed
-
- Barr, V.A., and A.L. Hubbard. 1993. Newly synthesized hepatocyte PM proteins are transported in transcytotic vesicles in the bile duct-ligated rat. Gastroenterology. 105:554–571. - PubMed
-
- Barr, V.A., L.J. Scott, and A.L. Hubbard. 1995. Immunoadsorption of hepatic vesicles carrying newly synthesized dipeptidyl peptidase IV and polymeric IgA receptor. J. Biol. Chem. 46:27834–27844. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

