Human STAGA complex is a chromatin-acetylating transcription coactivator that interacts with pre-mRNA splicing and DNA damage-binding factors in vivo
- PMID: 11564863
- PMCID: PMC99856
- DOI: 10.1128/MCB.21.20.6782-6795.2001
Human STAGA complex is a chromatin-acetylating transcription coactivator that interacts with pre-mRNA splicing and DNA damage-binding factors in vivo
Abstract
GCN5 is a histone acetyltransferase (HAT) originally identified in Saccharomyces cerevisiae and required for transcription of specific genes within chromatin as part of the SAGA (SPT-ADA-GCN5 acetylase) coactivator complex. Mammalian cells have two distinct GCN5 homologs (PCAF and GCN5L) that have been found in three different SAGA-like complexes (PCAF complex, TFTC [TATA-binding-protein-free TAF(II)-containing complex], and STAGA [SPT3-TAF(II)31-GCN5L acetylase]). The composition and roles of these mammalian HAT complexes are still poorly characterized. Here, we present the purification and characterization of the human STAGA complex. We show that STAGA contains homologs of most yeast SAGA components, including two novel human proteins with histone-like folds and sequence relationships to yeast SPT7 and ADA1. Furthermore, we demonstrate that STAGA has acetyl coenzyme A-dependent transcriptional coactivator functions from a chromatin-assembled template in vitro and associates in HeLa cells with spliceosome-associated protein 130 (SAP130) and DDB1, two structurally related proteins. SAP130 is a component of the splicing factor SF3b that associates with U2 snRNP and is recruited to prespliceosomal complexes. DDB1 (p127) is a UV-damaged-DNA-binding protein that is involved, as part of a complex with DDB2 (p48), in nucleotide excision repair and the hereditary disease xeroderma pigmentosum. Our results thus suggest cellular roles of STAGA in chromatin modification, transcription, and transcription-coupled processes through direct physical interactions with sequence-specific transcription activators and with components of the splicing and DNA repair machineries.
Figures



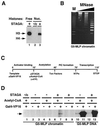
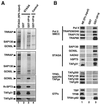
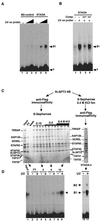
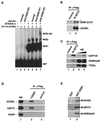
Similar articles
-
UV-damaged DNA-binding protein in the TFTC complex links DNA damage recognition to nucleosome acetylation.EMBO J. 2001 Jun 15;20(12):3187-96. doi: 10.1093/emboj/20.12.3187. EMBO J. 2001. PMID: 11406595 Free PMC article.
-
MYC interacts with the human STAGA coactivator complex via multivalent contacts with the GCN5 and TRRAP subunits.Biochim Biophys Acta. 2014 May;1839(5):395-405. doi: 10.1016/j.bbagrm.2014.03.017. Epub 2014 Apr 3. Biochim Biophys Acta. 2014. PMID: 24705139 Free PMC article.
-
Human ATAC Is a GCN5/PCAF-containing acetylase complex with a novel NC2-like histone fold module that interacts with the TATA-binding protein.J Biol Chem. 2008 Dec 5;283(49):33808-15. doi: 10.1074/jbc.M806936200. Epub 2008 Oct 6. J Biol Chem. 2008. PMID: 18838386 Free PMC article.
-
Recruitment of chromatin remodelling factors during gene activation via the glucocorticoid receptor N-terminal domain.Biochem Soc Trans. 2000;28(4):410-4. Biochem Soc Trans. 2000. PMID: 10961930 Review.
-
Acetylation of histones and transcription-related factors.Microbiol Mol Biol Rev. 2000 Jun;64(2):435-59. doi: 10.1128/MMBR.64.2.435-459.2000. Microbiol Mol Biol Rev. 2000. PMID: 10839822 Free PMC article. Review.
Cited by
-
Dynamic modules of the coactivator SAGA in eukaryotic transcription.Exp Mol Med. 2020 Jul;52(7):991-1003. doi: 10.1038/s12276-020-0463-4. Epub 2020 Jul 3. Exp Mol Med. 2020. PMID: 32616828 Free PMC article. Review.
-
Inhibition of tumorigenesis by the thyroid hormone receptor β in xenograft models.Thyroid. 2014 Feb;24(2):260-9. doi: 10.1089/thy.2013.0054. Epub 2013 Sep 4. Thyroid. 2014. PMID: 23731250 Free PMC article.
-
Sequential binding of UV DNA damage binding factor and degradation of the p48 subunit as early events after UV irradiation.Nucleic Acids Res. 2002 Jun 1;30(11):2588-98. doi: 10.1093/nar/30.11.2588. Nucleic Acids Res. 2002. PMID: 12034848 Free PMC article.
-
Analysis of Spt7 function in the Saccharomyces cerevisiae SAGA coactivator complex.Mol Cell Biol. 2002 Aug;22(15):5367-79. doi: 10.1128/MCB.22.15.5367-5379.2002. Mol Cell Biol. 2002. PMID: 12101232 Free PMC article.
-
Pygo2 associates with MLL2 histone methyltransferase and GCN5 histone acetyltransferase complexes to augment Wnt target gene expression and breast cancer stem-like cell expansion.Mol Cell Biol. 2010 Dec;30(24):5621-35. doi: 10.1128/MCB.00465-10. Epub 2010 Oct 11. Mol Cell Biol. 2010. PMID: 20937768 Free PMC article.
References
-
- Aboussekhra A, Biggerstaff M, Shivji M K, Vilpo J A, Moncollin V, Podust V N, Protic M, Hubscher U, Egly J M, Wood R D. Mammalian DNA nucleotide excision repair reconstituted with purified protein components. Cell. 1995;80:859–868. - PubMed
-
- Agalioti T, Lomvardas S, Parekh B, Yie J, Maniatis T, Thanos D. Ordered recruitment of chromatin modifying and general transcription factors to the IFN-β promoter. Cell. 2000;103:667–678. - PubMed
-
- Ayer D E. Histone deacetylases: transcriptional repression with SINers and NuRDs. Trends Cell Biol. 1999;9:193–198. - PubMed
-
- Becker P B, Tsukiyama T, Wu C. Chromatin assembly extracts from Drosophila embryos. Methods Cell Biol. 1994;44:207–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
