Measuring virulence factor expression by the pathogenic bacterium Photorhabdus luminescens in culture and during insect infection
- PMID: 11566980
- PMCID: PMC99659
- DOI: 10.1128/JB.183.20.5834-5839.2001
Measuring virulence factor expression by the pathogenic bacterium Photorhabdus luminescens in culture and during insect infection
Abstract
During insect infection Photorhabdus luminescens emits light and expresses virulence factors, including insecticidal toxin complexes (Tcs) and an RTX-like metalloprotease (Prt). Using quantitative PCR and protein assays, we describe the expression patterns of these factors both in culture and during insect infection and compare them to the associated bacterial growth curves. In culture, light and active Prt protease are produced in stationary phase. Tca also appears in stationary phase, whereas Tcd is expressed earlier. These patterns seen in a culture flask are strikingly similar to those observed during insect infection. Thus, in an infected insect, bacteria grow exponentially until the time of insect death at approximately 48 h, when both light and the virulence factors Prt protease and Tca are produced. In contrast, Tcd appears much earlier in insect infection. However, at present, the biological significance of this difference in timing of the production of the two toxins in unclear. This is the first documentation of the expression of Tcs and Prt in an insect and highlights the malleability of Photorhabdus as a model system for bacterial infection.
Figures
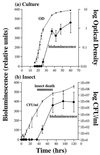
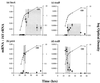


References
-
- Bowen D, Blackburn M, Rocheleau T, Grutzmacher C, ffrench-Constant R H. Secreted proteases from Photorhabdus luminescens: separation of the extracellular proteases from the insecticidal Tc toxin complexes. Insect Biochem Mol Biol. 2000;30:69–74. - PubMed
-
- Bowen D, Rocheleau T A, Blackburn M, Andreev O, Golubeva E, Bhartia R, ffrench-Constant R H. Insecticidal toxins from the bacterium Photorhabdus luminescens. Science. 1998;280:2129–2132. - PubMed
-
- Ehlers R U, Niemann I. Molecular identification of Photorhabdus luminescens strains by amplification of specific fragments of the 16S ribosomal DNA. Syst Appl Microbiol. 1998;21:509–519. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

