GerN, an endospore germination protein of Bacillus cereus, is an Na(+)/H(+)-K(+) antiporter
- PMID: 11566988
- PMCID: PMC99667
- DOI: 10.1128/JB.183.20.5896-5903.2001
GerN, an endospore germination protein of Bacillus cereus, is an Na(+)/H(+)-K(+) antiporter
Abstract
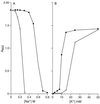
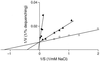
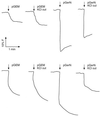
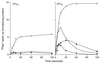
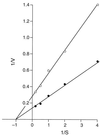
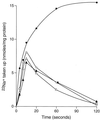
GerN, a Bacillus cereus spore germination protein, exhibits homology to a widely distributed group of putative cation transporters or channel proteins. GerN complemented the Na(+)-sensitive phenotype of an Escherichia coli mutant that is deficient in Na(+)/H(+) antiport activity (strain KNabc). GerN also reduced the concentration of K(+) required to support growth of an E. coli mutant deficient in K(+) uptake (strain TK2420). In a fluorescence-based assay of everted E. coli KNabc membrane vesicles, GerN exhibited robust Na(+)/H(+) antiport activity, with a K(m) for Na(+) estimated at 1.5 mM at pH 8.0 and 25 mM at pH 7.0. Li(+), but not K(+), served as a substrate. GerN-mediated Na(+)/H(+) antiport was further demonstrated in everted vesicles as energy-dependent accumulation of (22)Na(+). GerN also used K(+) as a coupling ion without completely replacing H(+), as indicated by partial inhibition by K(+) of H(+) uptake into right-side-out vesicles loaded with Na(+). K(+) translocation as part of the antiport was supported by the stimulatory effect of intravesicular K(+) on (22)Na(+) uptake by everted vesicles and the dependence of GerN-mediated (86)Rb(+) efflux on the presence of Na(+) in trans. The inhibitory patterns of protonophore and thiocyanate were most consistent with an electrogenic Na(+)/H(+)-K(+) antiport. GerN-mediated Na(+)/H(+)-K(+) antiport was much more rapid than GerN-mediated Na(+)/H(+) antiport.
Figures



References
-
- Ambudkar S V, Zlotnick G U, Rosen B P. Calcium efflux from Escherichia coli: evidence for two systems. J Biol Chem. 1984;259:6142–6146. - PubMed
-
- Booth I R, Jones M A, McLaggan D, Nikolaev Y, Ness L S, Wood C M, Miller S, Totemeyer S, Ferguson G P. Bacterial ion channels. In: Konings W N, Kaback H R, Lolkema J S, editors. Handbook of biological physics. Vol. 2. Amsterdam, The Netherlands: Elsevier Science; 1996. pp. 693–729.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

