Oxidative glutamate toxicity can be a component of the excitotoxicity cascade
- PMID: 11567035
- PMCID: PMC6762876
- DOI: 10.1523/JNEUROSCI.21-19-07455.2001
Oxidative glutamate toxicity can be a component of the excitotoxicity cascade
Abstract
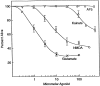
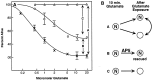
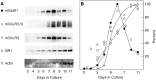
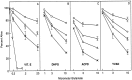
Along with ionotropic and metabotropic glutamate receptors, the cystine/glutamate antiporter x(c)(-) may play a critical role in CNS pathology. High levels of extracellular glutamate inhibit the import of cystine, resulting in the depletion of glutathione and a form of cell injury called oxidative glutamate toxicity. Here we show that a portion of the cell death associated with NMDA receptor-initiated excitotoxicity can be caused by oxidative glutamate toxicity. In primary mouse cortical neurons the cell death resulting from the short-term application of 10 microm glutamate can be divided into NMDA and NMDA receptor-independent phases. The NMDA receptor-independent component is associated with high extracellular glutamate and is inhibited by a variety of reagents that block oxidative glutamate toxicity. These results suggest that oxidative glutamate toxicity toward neurons lacking functional NMDA receptors can be a component of the excitotoxicity-initiated cell death pathway.
Figures


References
-
- Akazawa C, Shigemoto R, Bessho Y, Nakanishi S, Mizuno N. Differential expression of five N-methyl-d-aspartate receptor subunit mRNAs in the cerebellum of developing and adult rats. J Comp Neurol. 1994;347:150–160. - PubMed
-
- Ankacrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. - PubMed
-
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
