Cns distribution of members of the two-pore-domain (KCNK) potassium channel family
- PMID: 11567039
- PMCID: PMC6762917
- DOI: 10.1523/JNEUROSCI.21-19-07491.2001
Cns distribution of members of the two-pore-domain (KCNK) potassium channel family
Abstract
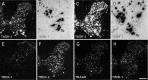
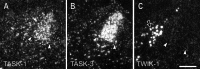
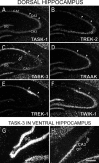
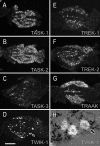
Two-pore-domain potassium (K(+)) channels are substrates for resting K(+) currents in neurons. They are major targets for endogenous modulators, as well as for clinically important compounds such as volatile anesthetics. In the current study, we report on the CNS distribution in the rat and mouse of mRNA encoding seven two-pore-domain K(+) channel family members: TASK-1 (KCNK3), TASK-2 (KCNK5), TASK-3 (KCNK9), TREK-1 (KCNK2), TREK-2 (KCNK10), TRAAK (KCNK4), and TWIK-1 (KCNK1). All of these genes were expressed in dorsal root ganglia, and for all of the genes except TASK-2, there was a differential distribution in the CNS. For TASK-1, highest mRNA accumulation was seen in the cerebellum and somatic motoneurons. TASK-3 was much more widely distributed, with robust expression in all brain regions, with particularly high expression in somatic motoneurons, cerebellar granule neurons, the locus ceruleus, and raphe nuclei and in various nuclei of the hypothalamus. TREK-1 was highest in the striatum and in parts of the cortex (layer IV) and hippocampus (CA2 pyramidal neurons). mRNA for TRAAK also was highest in the cortex, whereas expression of TREK-2 was primarily restricted to the cerebellar granule cell layer. There was widespread distribution of TWIK-1, with highest levels in the cerebellar granule cell layer, thalamic reticular nucleus, and piriform cortex. The differential expression of each of these genes likely contributes to characteristic excitability properties in distinct populations of neurons, as well as to diversity in their susceptibility to modulation.
Figures





References
-
- Bang H, Kim Y, Kim D. TREK-2, a new member of the mechanosensitive tandem-pore K+ channel family. J Biol Chem. 2000;275:17412–17419. - PubMed
-
- Bearzatto B, Lesage F, Reyes R, Lazdunski M, Laduron PM. Axonal transport of TREK and TRAAK potassium channels in rat sciatic nerves. NeuroReport. 2000;11:927–930. - PubMed
-
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. - PubMed
-
- Chapman CG, Meadows HJ, Godden RJ, Campbell DA, Duckworth M, Kelsell RE, Murdock PR, Randall AD, Rennie GI, Gloger IS. Cloning, localisation and functional expression of a novel human, cerebellum specific, two pore domain potassium channel. Brain Res Mol Brain Res. 2000;82:74–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
