Contactin associates with Na+ channels and increases their functional expression
- PMID: 11567041
- PMCID: PMC6762905
- DOI: 10.1523/JNEUROSCI.21-19-07517.2001
Contactin associates with Na+ channels and increases their functional expression
Abstract
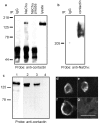
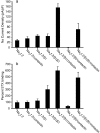
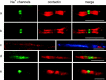
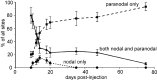
Contactin (also known as F3, F11) is a surface glycoprotein that has significant homology with the beta2 subunit of voltage-gated Na(+) channels. Contactin and Na(+) channels can be reciprocally coimmunoprecipitated from brain homogenates, indicating association within a complex. Cells cotransfected with Na(+) channel Na(v)1.2alpha and beta1 subunits and contactin have threefold to fourfold higher peak Na(+) currents than cells with Na(v)1.2alpha alone, Na(v)1.2/beta1, Na(v)1.2/contactin, or Na(v)1.2/beta1/beta2. These cells also have a correspondingly higher saxitoxin binding, suggesting an increased Na(+) channel surface membrane density. Coimmunoprecipitation of different subunits from cell lines shows that contactin interacts specifically with the beta1 subunit. In the PNS, immunocytochemical studies show a transient colocalization of contactin and Na(+) channels at new nodes of Ranvier forming during remyelination. In the CNS, there is a particularly high level of colocalization of Na(+) channels and contactin at nodes both during development and in the adult. Contactin may thus significantly influence the functional expression and distribution of Na(+) channels in neurons.
Figures


References
-
- Bennett V, Lambert S. Physiological roles of axonal ankyrins in survival of premyelinated axons and localization of voltage-gated sodium channels. J Neurocytol. 1999;28:303–318. - PubMed
-
- Berglund EO, Murai KK, Fredette B, Sekerkova G, Marturano B, Weber L, Mugnaini E, Ranscht B. Ataxia and abnormal cerebellar microorganization in mice with ablated contactin gene expression. Neuron. 1999;24:739–750. - PubMed
-
- Berglund EO, Boyle MET, Murai KK, Peles E, Weber L, Ranscht B. Contactin regulates axon–Schwann cell interactions at the paranode in myelinated peripheral nerve. Soc Neurosci Abstr. 2000;26:1081.
-
- Brummendorf T, Wolff JM, Frank R, Rathjen FG. Neural cell recognition molecule F11: homology with fibronectin type III and immunoglobulin type C domains. Neuron. 1989;2:1351–1361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
