Estrogen and Bcl-2: gene induction and effect of transgene in experimental stroke
- PMID: 11567044
- PMCID: PMC6762919
- DOI: 10.1523/JNEUROSCI.21-19-07543.2001
Estrogen and Bcl-2: gene induction and effect of transgene in experimental stroke
Abstract
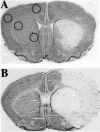
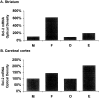
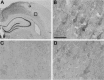
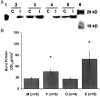
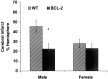
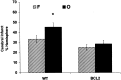
Female rodents producing endogenous estrogens are protected from stroke damage in comparison with male counterparts. This natural protection is lost after ovariectomy or reproductive senescence. The aim of this study is to determine whether estrogen reduces early neuronal injury and cell loss after ischemia by increasing the expression of Bcl-2. Male, intact female, ovariectomized, and estrogen-repleted ovariectomized rats were subjected to middle cerebral artery occlusion, and 22 hr later the level and localization of Bcl-2 mRNA and protein were determined. The levels of post-ischemic bcl-2 mRNA and protein were increased exclusively in neurons within the peri-infarct region. Intact females and estrogen-treated castrates demonstrated increased bcl-2 mRNA and protein expression compared with males and estrogen-deficient females, accompanied by a decrease in infarct size. To test the hypothesis that the neuroprotective mechanism of estrogen functions via Bcl-2, we compared ischemic outcome in male, female, and ovariectomized wild-type mice and mice overexpressing Bcl-2 exclusively in neurons. Wild-type female mice sustained smaller infarcts compared with males. Bcl-2 overexpression reduced infarct size in males, but provided no added protection in the female. Moreover, ovariectomy exacerbated infarction in wild-type females, but had no effect in Bcl-2 overexpressors. These data indicate that overexpression of Bcl-2 simulates the protection against ischemic injury conferred by endogenous female sex steroids. We concluded that estrogen rescues neurons after focal cerebral ischemia by increasing the level of Bcl-2 in peri-infarct regions and that estrogen-induced bcl-2 gene expression is an important downstream component of neuronal protection in female stroke.
Figures

References
-
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998a;29:159–165. - PubMed
-
- Alkayed NJ, Sharpe LG, McCune SK, Crain BJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection is associated with higher postischemic expression of bcl-2 mRNA in rat brain. Soc Neurosci Abstr. 1998b;24:517.
-
- Alkayed NJ, Murphy JM, Traystman RJ, Hurn PD. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31:161–168. - PubMed
-
- Antonawich FJ, Federoff HJ, Davis JN. Bcl-2 transduction, using a herpes simplex virus amplicon, protects hippocampal neurons from transient global ischemia. Exp Neurol. 1999;156:130–137. - PubMed
-
- Bernard R, Farlie P, Bernard O. NSE-bcl-2 transgenic mice, a model system for studying neuronal death and survival. Dev Neurosci. 1997;19:79–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
