Glutamate blocks serotonergic phase advances of the mammalian circadian pacemaker through AMPA and NMDA receptors
- PMID: 11567072
- PMCID: PMC6762886
- DOI: 10.1523/JNEUROSCI.21-19-07815.2001
Glutamate blocks serotonergic phase advances of the mammalian circadian pacemaker through AMPA and NMDA receptors
Abstract
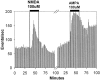
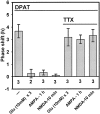
The phase of the mammalian circadian pacemaker, located in the suprachiasmatic nucleus (SCN), is modulated by a variety of stimuli, most notably the environmental light cycle. Light information is perceived by the circadian pacemaker through glutamate that is released from retinal ganglion cell terminals in the SCN. Other prominent modulatory inputs to the SCN include a serotonergic projection from the raphe nuclei and a neuropeptide Y (NPY) input from the intergeniculate leaflet. Light and glutamate phase-shift the SCN pacemaker at night, whereas serotonin (5-HT) and NPY primarily phase-shift the pacemaker during the day. In addition to directly phase-shifting the circadian pacemaker, SCN inputs have been shown to modulate the actions of one another. For example, 5-HT can inhibit the phase-shifting effects of light or glutamate applied to the SCN at night, and NPY and glutamate inhibit phase shifts of one another. In this study, we explored the possibility that glutamate can modulate serotonergic phase shifts during the day. For these experiments, we applied various combinations of 5-HT agonists, glutamate agonists, and electrical stimulation of the optic chiasm to SCN brain slices to determine the effect of these treatments on the rhythm of spontaneous neuronal activity generated by the SCN circadian pacemaker. We found that glutamate agonists and optic chiasm stimulation inhibit serotonergic phase advances and that this inhibition involves both AMPA and NMDA receptors. This inhibition by glutamate may be indirect, because it is blocked by both tetrodotoxin and the GABA(A) antagonist, bicuculline.
Figures





Similar articles
-
Melatonin inhibits in vitro serotonergic phase shifts of the suprachiasmatic circadian clock.Brain Res. 1999 Feb 13;818(2):408-13. doi: 10.1016/s0006-8993(98)01295-5. Brain Res. 1999. PMID: 10082826
-
Enhanced NMDA receptor activity in retinal inputs to the rat suprachiasmatic nucleus during the subjective night.J Physiol. 2001 Apr 1;532(Pt 1):181-94. doi: 10.1111/j.1469-7793.2001.0181g.x. J Physiol. 2001. PMID: 11283234 Free PMC article.
-
Serotonin and the mammalian circadian system: I. In vitro phase shifts by serotonergic agonists and antagonists.J Biol Rhythms. 1993 Spring;8(1):1-16. doi: 10.1177/074873049300800101. J Biol Rhythms. 1993. PMID: 8490207
-
Serotonergic innervation of the hypothalamic suprachiasmatic nucleus and photic regulation of circadian rhythms.Biol Cell. 1997 Nov;89(8):513-23. doi: 10.1016/s0248-4900(98)80007-5. Biol Cell. 1997. PMID: 9618901 Review.
-
Serotonin and the regulation of mammalian circadian rhythmicity.Ann Med. 1999 Feb;31(1):12-33. doi: 10.3109/07853899909019259. Ann Med. 1999. PMID: 10219711 Review.
Cited by
-
Acute ethanol modulates glutamatergic and serotonergic phase shifts of the mouse circadian clock in vitro.Neuroscience. 2008 Mar 27;152(3):837-48. doi: 10.1016/j.neuroscience.2007.12.049. Epub 2008 Jan 29. Neuroscience. 2008. PMID: 18313227 Free PMC article.
-
Intrinsic role of polysialylated neural cell adhesion molecule in photic phase resetting of the Mammalian circadian clock.J Neurosci. 2003 Jan 15;23(2):652-8. doi: 10.1523/JNEUROSCI.23-02-00652.2003. J Neurosci. 2003. PMID: 12533624 Free PMC article.
-
Circadian clock resetting in the mouse changes with age.Age (Dordr). 2009 Dec;31(4):293-303. doi: 10.1007/s11357-009-9102-7. Age (Dordr). 2009. PMID: 19557547 Free PMC article.
-
The Neurotransmitters Involved in Drosophila Alcohol-Induced Behaviors.Front Behav Neurosci. 2020 Dec 15;14:607700. doi: 10.3389/fnbeh.2020.607700. eCollection 2020. Front Behav Neurosci. 2020. PMID: 33384590 Free PMC article. Review.
-
Circadian entrainment and its role in depression: a mechanistic review.J Neural Transm (Vienna). 2012 Oct;119(10):1085-96. doi: 10.1007/s00702-012-0858-z. Epub 2012 Jul 14. J Neural Transm (Vienna). 2012. PMID: 22798027 Review.
References
-
- Akiyama M, Kouzu Y, Takahashi S, Wakamatsu H, Moriya T, Maetani M, Watanabe S, Tei H, Sakaki Y, Shibata S. Inhibition of light- or glutamate-induced mPer2 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J Neurosci. 1999;19:1115–1121. - PMC - PubMed
-
- Antle MC, Marchant EG, Niel L, Mistlberger RE. Serotonin antagonists do not attenuate activity-induced phase shifts of circadian rhythms in the Syrian hamster. Brain Res. 1998;813:139–149. - PubMed
-
- Bergeron HE, Danielson B, Biggs KR, Prosser RA. TTX blocks baclofen-induced phase shifts of the mammalian circadian pacemaker in vitro. Brain Res. 1999;841:193–196. - PubMed
-
- Biello SM. Enhanced photic phase shifting after treatment with antiserum to neuropeptide Y. Brain Res. 1995;673:25–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
