Multistep nucleus formation and a separate subunit contribution of the amyloidgenesis of heat-denatured monellin
- PMID: 11567100
- PMCID: PMC2374219
- DOI: 10.1110/ps.20201
Multistep nucleus formation and a separate subunit contribution of the amyloidgenesis of heat-denatured monellin
Abstract
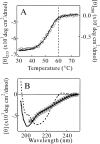
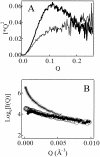
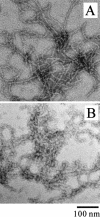
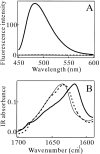
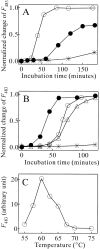
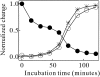
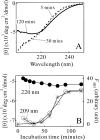
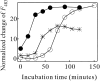
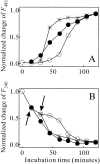
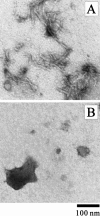
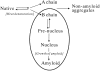
Monellin (MN) is a sweet-tasting plant protein known to form fibrous aggregates in the heat-denatured state. Here the amyloid-type aggregation process of MN is extensively characterized. The amyloidgenesis was initiated in a highly denatured state of MN. A seeding effect of skipping a lag phase of the amyloid formation kinetics established a nucleation-dependent aggregation mechanism. A finely controlled experimental protocol revealed an additional prenucleus stage preceding the maturation of the nucleus, indicating that the initial lag phase is composed of multiple conformational events. The results obtained for the aggregation properties of the separate A and B subunit chains of MN and a recombinant single-chain MN suggest that the B chain exclusively contributed to the amyloid-type aggregation. These findings suggest a scheme for the amyloidgenesis of MN and their subunits, and provide a unique model of amyloidgenesis that is regulated by the subunit composition of protein.
Figures
References
-
- Bjellqvist, B., Hughes, G.J., Pasquali, C., Paquet, N., Ravier, F., Sanchez, J.C., Frutiger, S., and Hochstrasser, D. 1993. The focusing positions of polypeptides in immobilized pH gradients can be predicted from their amino acid sequences. Electrophoresis 14 1023–1031. - PubMed
-
- Dobson, C.M. and Karplus, M. 1999. The fundamentals of protein folding: Bringing together theory and experiment. Curr. Opin. Struct. Biol. 9 92–101. - PubMed
-
- Fan, P., Bracken, C., and Baum, J. 1993. Structural characterization of monellin in the alcohol-denatured state by NMR: Evidence for β-sheet to α-helix conversion. Biochemistry 32 1573–1582. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

