Motility-related proteins as markers for head and neck squamous cell cancer
- PMID: 11568556
- PMCID: PMC3616334
- DOI: 10.1097/00005537-200107000-00027
Motility-related proteins as markers for head and neck squamous cell cancer
Abstract
Hypothesis: Increased cell motility is a hallmark of cancer cells. Proteins involved in cell motility may be used as molecular markers to characterize the malignant potential of tumors.
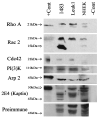
Methods: Molecular biology and immunohistochemistry techniques were used to investigate the expression of a selected panel of motility-related proteins (Rho A, Rac 2, Cdc42, PI3K, 2E4, and Arp2) in normal, premalignant, and squamous cell cancer cell lines of human head and neck origin. To assess the clinical potential of these proteins as molecular markers for cancer, immunohistochemistry was performed on paraffin-fixed head and neck cancer specimens (n = 15).
Results: All six motility-associated proteins were overexpressed in the premalignant and squamous cell cancer cell lines relative to normal keratinocytes. Immunohistochemistry with Rho A and Rac 2 showed increased staining in areas of cancer but not in normal tissue.
Conclusion: Proteins involved in cell motility can be used as markers for head and neck squamous cell carcinoma. The head and neck cell lines used in this study may be used as a model to further investigate cell motility. Molecular markers of motility could have a significant impact on the diagnosis and staging of cancers originating from differentiated non-motile cells.
Figures





References
-
- Liotta L. Mechanisms of cancer invasion and metastasis. In: DeVita VT, Hellman A, Rosenberg S, editors. Progress in Oncology. vol 1. Lippincott; New York: 1985. pp. 28–42.
-
- Cunningham CC. Actin structural proteins in cell motility. Cancer Metastasis Rev. 1992;11:69–77. - PubMed
-
- Ridley AJ. The small GTP-binding rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. - PubMed
-
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein Rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

