Presynaptic kainate receptors at hippocampal mossy fiber synapses
- PMID: 11572960
- PMCID: PMC58674
- DOI: 10.1073/pnas.191351498
Presynaptic kainate receptors at hippocampal mossy fiber synapses
Abstract
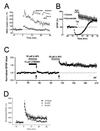
Hippocampal mossy fibers, which are the axons of dentate granule cells, form powerful excitatory synapses onto the proximal dendrites of CA3 pyramidal cells. It has long been known that high-affinity binding sites for kainate, a glutamate receptor agonist, are present on mossy fibers. Here we summarize recent experiments on the role of these presynaptic kainate receptors (KARs). Application of kainate has a direct effect on the amplitude of the extracellularly recorded fiber volley, with an enhancement by low concentrations and a depression by high concentrations. These effects are mediated by KARs, because they persist in the presence of the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-selective antagonist GYKI 53655, but are blocked by the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/KAR antagonist 6-cyano-7-nitroquinoxaline-2,3-dione and the KAR antagonist SYM2081. The effects on the fiber volley are most likely caused by a depolarization of the fibers via the known ionotropic actions of KARs, because application of potassium mimics the effects. In addition to these effects on fiber excitability, low concentrations of kainate enhance transmitter release, whereas high concentrations depress transmitter release. Importantly, the synaptic release of glutamate from mossy fibers also activates these presynaptic KARs, causing an enhancement of the fiber volley and a facilitation of release that lasts for many seconds. This positive feedback contributes to the dramatic frequency facilitation that is characteristic of mossy fiber synapses. It will be interesting to determine how widespread facilitatory presynaptic KARs are at other synapses in the central nervous system.
Figures







References
-
- Eccles J C. The Physiology of Synapses. Berlin: Springer; 1964.
-
- Nicoll R A, Alger B E. Int Rev Neurobiol. 1979;21:217–258. - PubMed
-
- Thompson S M, Capogna M, Scanziani M. Trends Neurosci. 1993;16:222–227. - PubMed
-
- Wu L G, Saggau P. Trends Neurosci. 1997;20:204–212. - PubMed
-
- MacDermott A B, Role L W, Siegelbaum S A. Annu Rev Neurosci. 1999;22:443–485. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

