Fruiting body formation by Bacillus subtilis
- PMID: 11572999
- PMCID: PMC58779
- DOI: 10.1073/pnas.191384198
Fruiting body formation by Bacillus subtilis
Abstract
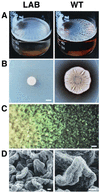
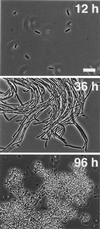
Spore formation by the bacterium Bacillus subtilis has long been studied as a model for cellular differentiation, but predominantly as a single cell. When analyzed within the context of highly structured, surface-associated communities (biofilms), spore formation was discovered to have heretofore unsuspected spatial organization. Initially, motile cells differentiated into aligned chains of attached cells that eventually produced aerial structures, or fruiting bodies, that served as preferential sites for sporulation. Fruiting body formation depended on regulatory genes required early in sporulation and on genes evidently needed for exopolysaccharide and surfactin production. The formation of aerial structures was robust in natural isolates but not in laboratory strains, an indication that multicellularity has been lost during domestication of B. subtilis. Other microbial differentiation processes long thought to involve only single cells could display the spatial organization characteristic of multicellular organisms when studied with recent natural isolates.
Figures


References
-
- Losick R, Shapiro L, editors. Microbial Development. Plainview, NY: Cold Spring Harbor Lab. Press; 1984.
-
- Dworkin M. Developmental Biology of the Bacteria. San Francisco: Benjamin/Cummings Science; 1985.
-
- Brun Y V, Shimkets L J, editors. Prokaryotic Development. Washington, DC: Am. Soc. Microbiol.; 2000.
-
- Piggot, P. & Losick, R. (in press) in Bacillus subtilis and Its Closest Relatives: From Genes to Cells, eds. Sonenshein, A. L., Hoch, J. A. & Losick, R. (Am. Soc. Microbiol., Washington, DC), in press.
-
- Setlow, P. (in press) in Bacillus subtilis and Its Closest Relatives: From Genes to Cells, eds. Sonenshein, A. L., Hoch, J. A. & Losick, R. (Am. Soc. Microbiol., Washington, DC), in press.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

