Initial recovery of vision after early monocular deprivation in kittens is faster when both eyes are open
- PMID: 11573003
- PMCID: PMC58786
- DOI: 10.1073/pnas.201392698
Initial recovery of vision after early monocular deprivation in kittens is faster when both eyes are open
Abstract
A comparison was made of the speed of visual recovery in the deprived eye of kittens after a 6-day period of monocular deprivation imposed at 5-9 weeks of age in two postdeprivation conditions. In one condition, binocular recovery (BR), both eyes were open, whereas in the other condition, reverse lid-suture (RLS), the formerly nondeprived eye was closed to force the animal to use the originally deprived eye. In littermate pairs, BR kittens began to recover form vision 12 to 30 h before those subjected to RLS. The vision of the deprived eye of the BR animals remained superior to that of their RLS littermates for 4-8 days. Although this finding is difficult to reconcile with competitive mechanisms of synaptic plasticity, it supports a prediction of an alternative model of synaptic plasticity [Bienenstock, E. L., Cooper, L. N. & Munro, P. W. (1982) J. Neurosci. 2, 32-48] for slower initial recovery with RLS because of the time required to reset the modification threshold.
Figures

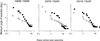

References
-
- Wiesel T N, Hubel D H. J Neurophysiol. 1965;28:1029–1040. - PubMed
-
- Guillery R W. In: The Making of the Nervous System. Parnevelas J G, Stern C J, Sterling RV, editors. Oxford: Oxford Univ. Press; 1988. pp. 356–379.
-
- Hubel D H, Wiesel T N, LeVay S. Philos Trans R Soc London B. 1977;278:377–409. - PubMed
-
- Miller K D, Keller J B, Stryker M P. Science. 1989;245:605–615. - PubMed
-
- Mitchell D E, Cynader M, Movshon J A. J Comp Neurol. 1977;176:53–64. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

