A zinc-sensing receptor triggers the release of intracellular Ca2+ and regulates ion transport
- PMID: 11573009
- PMCID: PMC58801
- DOI: 10.1073/pnas.201193398
A zinc-sensing receptor triggers the release of intracellular Ca2+ and regulates ion transport
Abstract
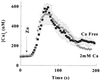
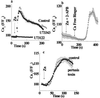
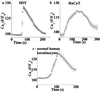
Changes in extracellular zinc concentration participate in modulating fundamental cellular processes such as proliferation, secretion, and ion transport in a mechanism that is not well understood. Here, we show that a micromolar concentration of extracellular zinc triggers a massive release of calcium from thapsigargin-sensitive intracellular pools in the colonocytic cell line HT29. Calcium release was blocked by a phospholipase-C inhibitor, indicating that formation of inositol 1,4,5-triphosphate is required for zinc-dependent calcium release. Zinc influx was not observed, indicating that extracellular zinc triggered the release. The Ca(i)2+ release was zinc specific and could not be triggered by other heavy metals. Furthermore, zinc failed to activate the Ca(2+)-sensing receptor heterologously expressed in HEK293 cells. The zinc-induced Ca(i)2+ rise stimulated the activity of the Na(+)/H(+) exchanger in HT29 cells. Our results indicate that a previously uncharacterized extracellular, G protein-coupled, Zn(2+)-sensing receptor is functional in colonocytes. Because Ca(i)2+ rise is known to regulate key cellular and signal-transduction processes, the zinc-sensing receptor may provide the missing link between extracellular zinc concentration changes and the regulation of cellular processes.
Figures
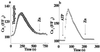



Comment in
-
Crosstalk of the group IIa and IIb metals calcium and zinc in cellular signaling.Proc Natl Acad Sci U S A. 2001 Oct 23;98(22):12325-7. doi: 10.1073/pnas.231481398. Proc Natl Acad Sci U S A. 2001. PMID: 11675482 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

