Interaction of coxsackievirus B3 with the full length coxsackievirus-adenovirus receptor
- PMID: 11573093
- PMCID: PMC4152846
- DOI: 10.1038/nsb1001-874
Interaction of coxsackievirus B3 with the full length coxsackievirus-adenovirus receptor
Abstract
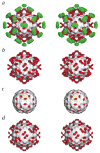
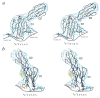
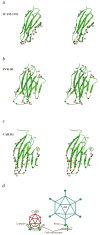
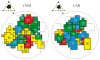
Group B coxsackieviruses (CVB) utilize the coxsackievirus-adenovirus receptor (CAR) to recognize host cells. CAR is a membrane protein with two Ig-like extracellular domains (D1 and D2), a transmembrane domain and a cytoplasmic domain. The three-dimensional structure of coxsackievirus B3 (CVB3) in complex with full length human CAR and also with the D1D2 fragment of CAR were determined to approximately 22 A resolution using cryo-electron microscopy (cryo-EM). Pairs of transmembrane domains of CAR associate with each other in a detergent cloud that mimics a cellular plasma membrane. This is the first view of a virus-receptor interaction at this resolution that includes the transmembrane and cytoplasmic portion of the receptor. CAR binds with the distal end of domain D1 in the canyon of CVB3, similar to how other receptor molecules bind to entero- and rhinoviruses. The previously described interface of CAR with the adenovirus knob protein utilizes a side surface of D1.
Figures
References
-
- Rueckert RR. In: Fields virology. Fields BN, Knipe DM, Howley PM, editors. Lippincott-Raven Press; Philadelphia & New York: 1996. pp. 609–654.
-
- Melnick JL. In: Fields virology. Fields BN, Knipe DM, Howley PM, editors. Lippincott-Raven Publishers; Philadelphia & New York: 1996. pp. 655–712.
-
- Muckelbauer JK, et al. Structure. 1995;3:653–668. - PubMed
-
- Lonberg-Holm K, Crowell RL, Philipson L. Nature. 1976;259:679–681. - PubMed
-
- Bergelson JM, et al. Science. 1997;275:1320–1323. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
