Reassignment of specificities of two cap methyltransferase domains in the reovirus lambda 2 protein
- PMID: 11574057
- PMCID: PMC56899
- DOI: 10.1186/gb-2001-2-9-research0038
Reassignment of specificities of two cap methyltransferase domains in the reovirus lambda 2 protein
Abstract
Background: The reovirus lambda2 protein catalyzes mRNA capping, that is, addition of a guanosine to the 5' end of each transcript in a 5'-to-5' orientation, as well as transfer of a methyl group from S-adenosyl-L-methionine (AdoMet) to the N7 atom of the added guanosyl moiety and subsequently to the ribose 2'-O atom of the first template-encoded nucleotide. The structure of the human reovirus core has been solved at 3.6 A resolution, revealing a series of domains that include a putative guanylyltransferase domain and two putative methyltransferase (MTase) domains. It has been suggested that the order of domains in the lambda2 protein corresponds to the order of reactions in the pathway and that the m7G (cap 0) and the 2'-O-ribose (cap 1) MTase activities may be exerted by the MTase 1 and the MTase 2 domains, respectively.
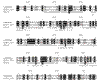
Results: We show that the reovirus MTase 1 domain shares a putative active site with the structurally characterized 2'-O-ribose MTases, including vaccinia virus cap 1 MTase, whereas the MTase 2 domain is structurally similar to glycine N-MTase.
Conclusions: On the basis of our analysis of the structural details we propose that the previously suggested functional assignments of the MTase 1 and MTase 2 domains should be swapped.
Figures
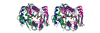

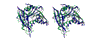

References
-
- Moss B, Gershowitz A, Wei CM, Boone R. Formation of the guanylylated and methylated 5'-terminus of vaccinia virus mRNA. Virology. 1976;72:341–351. - PubMed
-
- Furuichi Y, Muthukrishnan S, Tomasz J, Shatkin AJ. Mechanism of formation of reovirus mRNA 5'-terminal blocked and methylated sequence, m7GpppGmpC. J Biol Chem. 1976;251:5043–5053. - PubMed
-
- Varani G. A cap for all occasions. Structure. 1997;5:855–858. - PubMed
-
- Shuman S. Structure, mechanism, and evolution of the mRNA capping apparatus. Prog Nucleic Acid Res Mol Biol. 2000;66:1–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

