T-loop assembly in vitro involves binding of TRF2 near the 3' telomeric overhang
- PMID: 11574485
- PMCID: PMC125642
- DOI: 10.1093/emboj/20.19.5532
T-loop assembly in vitro involves binding of TRF2 near the 3' telomeric overhang
Abstract
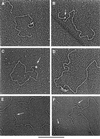
Mammalian telomeres contain a duplex TTAGGG-repeat tract terminating in a 3' single-stranded overhang. TRF2 protein has been implicated in remodeling telomeres into duplex lariats, termed t-loops, in vitro and t-loops have been isolated from cells in vivo. To examine the features of the telomeric DNA essential for TRF2-promoted looping, model templates containing a 500 bp double-stranded TTAGGG tract and ending in different single-stranded overhangs were constructed. As assayed by electron microscopy, looped molecules containing most of the telomeric tract are observed with TRF2 at the loop junction. A TTAGGG-3' overhang of at least six nucleotides is required for loop formation. Termini with 5' overhangs, blunt ends or 3' termini with non-telomeric sequences at the junction are deficient in loop formation. Addition of non-telomeric sequences to the distal portion of a 3' overhang beginning with TTAGGG repeats only modestly diminishes looping. TRF2 preferentially localizes to the junction between the duplex repeats and the single-stranded overhang. Based on these findings we suggest a model for the mechanism by which TRF2 remodels telomeres into t-loops.
Figures



References
-
- Bilaud T., Brun,C., Ancelin,K., Koering,C.E., Laroche,T. and Gilson,E. (1997) Telomeric localization of TRF2, a novel human telobox protein. Nature Genet., 17, 236–239. - PubMed
-
- Broccoli D., Smogorzewska,A., Chong,L. and de Lange,T. (1997) Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nature Genet., 17, 231–235. - PubMed
-
- Cacchione S., Cerone,M.A. and Savino,M. (1997) In vitro low propensity to form nucleosomes of four telomeric sequences. FEBS Lett., 400, 37–41. - PubMed
-
- Chong L., van Steensel,B., Broccoli,D., Erdjument-Bromage,H., Hanish, J., Tempst,P. and de Lange,T. (1995) A human telomeric protein. Science, 270, 1663–1667. - PubMed
-
- Cox M.M. (1994) Why does RecA protein hydrolyse ATP? Trends Biochem. Sci., 19, 217–222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

