Psoralen-modified clamp-forming antisense oligonucleotides reduce cellular c-Myc protein expression and B16-F0 proliferation
- PMID: 11574688
- PMCID: PMC60243
- DOI: 10.1093/nar/29.19.4052
Psoralen-modified clamp-forming antisense oligonucleotides reduce cellular c-Myc protein expression and B16-F0 proliferation
Abstract
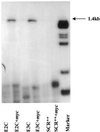
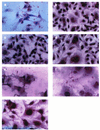
The c-myc protooncogene plays an important role in the abnormal growth pattern of melanoma cells. In an attempt to inhibit c-Myc expression and the growth of an established murine melanoma cell line, we targeted homopurine sequences within the mouse myc mRNA with modified antisense oligonucleotides (AS ODNs). Psoralen was conjugated to the 5'-end of these clamp-forming oligonucleotides (clamp ODNs). Gel mobility shift analysis demonstrated a sequence-specific interaction between the active clamp ODNs (Myc-E2C and Myc-E3C) and the 1.4 kb c-myc mRNA, but no interaction with the control clamp ODN (SCR**). This association was further confirmed by thermal denaturation studies. In vitro translation assays demonstrated that both Myc-E2C and Myc-E3C at 5 microM inhibited c-Myc expression >99% after UV activation at 366 nm. Immunostaining of B16-F0 cells with a c-Myc monoclonal antibody revealed a significant reduction in c-Myc after clamp ODN treatment compared with the untreated or SCR** control-treated cells. This result was corroborated by western blot analysis. Utilizing the MTT assay to determine the effects of ODN-mediated c-Myc reduction on B16-F0 growth, we observed 60 and 64% reductions in growth after treatment with 5 microM Myc-E3C and Myc-E2C, respectively. We attribute the enhanced effectiveness of the clamp ODNs to psoralen activation. Our preliminary data suggest that inhibiting c-Myc overexpression results in a significant reduction in abnormal proliferation of B16-F0 melanoma cells and that the increased efficiency of clamp ODNs may provide an important advantage for their use in antisense therapies.
Figures




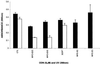
Similar articles
-
Acridine-modified, clamp-forming antisense oligonucleotides synergize with cisplatin to inhibit c-Myc expression and B16-F0 tumor progression.Nucleic Acids Res. 2002 Jun 1;30(11):2565-74. doi: 10.1093/nar/30.11.2565. Nucleic Acids Res. 2002. PMID: 12034846 Free PMC article.
-
Antitumor effect of c-myc antisense phosphorothioate oligodeoxynucleotides on human melanoma cells in vitro and and in mice.J Natl Cancer Inst. 1996 Apr 3;88(7):419-29. doi: 10.1093/jnci/88.7.419. J Natl Cancer Inst. 1996. PMID: 8618233
-
Antiproliferative effect of c-myc antisense phosphorothioate oligodeoxynucleotides in malignant glioma cells.Neurosurgery. 1997 Oct;41(4):908-15. doi: 10.1097/00006123-199710000-00027. Neurosurgery. 1997. PMID: 9316053
-
Rational design of sequence-specific oncogene inhibitors based on antisense and antigene oligonucleotides.Eur J Cancer. 1991;27(11):1466-71. doi: 10.1016/0277-5379(91)90033-a. Eur J Cancer. 1991. PMID: 1835863 Review.
-
Antisense of oligonucleotides and the inhibition of oncogene expression.Clin Oncol (R Coll Radiol). 1993;5(4):245-52. doi: 10.1016/s0936-6555(05)80238-9. Clin Oncol (R Coll Radiol). 1993. PMID: 8398922 Review.
Cited by
-
Acridine-modified, clamp-forming antisense oligonucleotides synergize with cisplatin to inhibit c-Myc expression and B16-F0 tumor progression.Nucleic Acids Res. 2002 Jun 1;30(11):2565-74. doi: 10.1093/nar/30.11.2565. Nucleic Acids Res. 2002. PMID: 12034846 Free PMC article.
References
-
- Kelly K. and Siebenlist,U. (1986) The regulation and expression of c-myc in normal and malignant cells. Annu. Rev. Immunol., 4, 317–338. - PubMed
-
- Marcu K.B., Bossone,S.A. and Patel,A.J. (1992) Myc function and regulation. Annu. Rev. Biochem., 61, 809–860. - PubMed
-
- Kim H.-G. and Miller,D.M. (1995) Inhibition of in vitro transcription by a triplex-forming oligonucleotide targeted to human c-myc P2 promoter. Biochemistry, 34, 8165–8171. - PubMed
-
- Cole M.D. (1986) The myc oncogene: its role in transformation and differentiation. Annu. Rev. Genet., 20, 361–384. - PubMed
-
- Bishop J.M. (1983) Cellular oncogenes and retroviruses. Annu. Rev. Biochem., 52, 301–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

