Nociceptin inhibits calcium channel currents in a subpopulation of small nociceptive trigeminal ganglion neurons in mouse
- PMID: 11579155
- PMCID: PMC2278836
- DOI: 10.1111/j.1469-7793.2001.t01-1-00035.x
Nociceptin inhibits calcium channel currents in a subpopulation of small nociceptive trigeminal ganglion neurons in mouse
Abstract
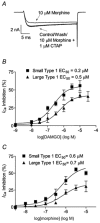
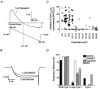
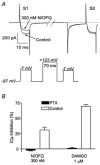
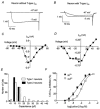
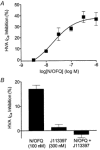
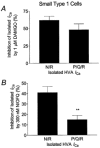
1. The effects of nociceptin/orphanin FQ (N/OFQ) and opioid receptor agonists on voltage-activated calcium channel currents (I(Ca)) were examined in acutely isolated mouse trigeminal ganglion neurons using whole-cell patch-clamp recordings. These effects were correlated with responses of the neurons to capsaicin and binding of Bandeiraea simplicifolia isolectin B4 (IB4). 2. Trigeminal neurons were divided into two populations based on the presence (type 2) or absence (type 1) of a prominent T-type I(Ca). N/OFQ potently (EC(50) of 19 nM) inhibited high-voltage-activated (HVA) I(Ca) in most (82 %) small (capacitance < 12 pF) type 1 neurons, but few (9 %) larger (> 12 pF) type 1 neurons. N/OFQ inhibited I(Ca) in few (23 %) type 2 cells, and did not affect the T-type I(Ca) in any cell. 3. The mu-opioid agonists DAMGO and morphine inhibited I(Ca) in most type 1 neurons, more often (95 % versus 77 %) in the small cells. The inhibition of I(Ca) by DAMGO and morphine was more efficacious in small versus large type 1 neurons. mu-Opioids did not inhibit I(Ca) in type 2 neurons. 4. Most small type 1 neurons were sensitive to capsaicin (93 %) and bound IB4 (86 %). Fewer larger type 1 neurons responded to capsaicin (30 %) or bound IB4 (58 %). Type 2 neurons did not respond to capsaicin, although some bound IB4 (35 %). 5. Thus, N/OFQ preferentially inhibits HVA I(Ca) in a subpopulation of small nociceptive trigeminal ganglion neurons that is also highly sensitive to mu-opioid agonists.
Figures

References
-
- Bourinet E, Soong TW, Sutton K, Slaymaker S, Matthews E, Monteil A, Zamponi GW, Nargeot J, Snutch TP. Splicing of α1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nature Neuroscience. 1999;2:407–415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

