Rapidly inactivating and non-inactivating calcium-activated potassium currents in frog saccular hair cells
- PMID: 11579156
- PMCID: PMC2278855
- DOI: 10.1111/j.1469-7793.2001.00049.x
Rapidly inactivating and non-inactivating calcium-activated potassium currents in frog saccular hair cells
Abstract
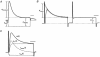
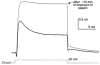
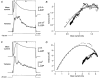
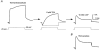
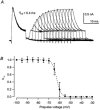
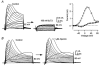
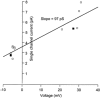
1. Using a semi-intact epithelial preparation we examined the Ca(2+)-activated K(+) (K(Ca)) currents of frog (Rana pipiens) saccular hair cells. After blocking voltage-dependent K(+) (K(V)) currents with 4-aminopyridine (4-AP) an outward current containing inactivating (I(transient)) and non-inactivating (I(steady)) components remained. 2. The contribution of each varied greatly from cell to cell, with I(transient) contributing from 14 to 90 % of the total outward current. Inactivation of I(transient) was rapid (tau approximately 2-3 ms) and occurred within the physiological range of membrane potentials (V(1/2) = -63 mV). Recovery from inactivation was also rapid (tau approximately 10 ms). 3. Suppression of both I(transient) and I(steady) by depolarizations that approached the Ca(2+) equilibrium potential and by treatments that blocked Ca(2+) influx (application Ca(2+)-free saline or Cd(2+)), suggest both are Ca(2+) dependent. Both were blocked by iberiotoxin, a specific blocker of large-conductance K(Ca) channels (BK), but not by apamin, a specific blocker of small-conductance K(Ca) channels. 4. Ensemble-variance analysis showed that I(transient) and I(steady) flow through two distinct populations of channels, both of which have a large single-channel conductance (~100 pS in non-symmetrical conditions). Together, these data indicate that both I(transient) and I(steady) are carried through BK channels, one of which undergoes rapid inactivation while the other does not. 5. Inactivation of I(transient) could be removed by extracellular papain and could later be restored by intracellular application of the 'ball' domain of the auxiliary subunit (beta2) thought to mediate BK channel inactivation in rat chromaffin cells. We hypothesize that I(transient) results from the association of a similar beta subunit with some of the BK channels and that papain removes inactivation by cleaving extracellular sites required for this association.
Figures
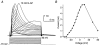
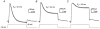

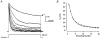
References
-
- Adelman JP, Shen KZ, Kavanaugh MP, Warren RA, Wu YN, Lagrutta A, Bond CT, North RA. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992;9:209–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

