Transcriptional transactivation by selected short random peptides attached to lexA-GFP fusion proteins
- PMID: 11580863
- PMCID: PMC56998
- DOI: 10.1186/1471-2199-2-10
Transcriptional transactivation by selected short random peptides attached to lexA-GFP fusion proteins
Abstract
Background: Transcriptional transactivation is a process with remarkable tolerance for sequence diversity and structural geometry. In studies of the features that constitute transactivating functions, acidity has remained one of the most common characteristics observed among native activation domains and activator peptides.
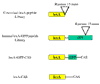
Results: We performed a deliberate search of random peptide libraries for peptides capable of conferring transcriptional transactivation on the lexA DNA binding domain. Two libraries, one composed of C-terminal fusions, the other of peptide insertions within the green fluorescent protein structure, were used. We show that (i) peptide sequences other than C-terminal fusions can confer transactivation; (ii) though acidic activator peptides are more common, charge neutral and basic peptides can function as activators; and (iii) peptides as short as 11 amino acids behave in a modular fashion.
Conclusions: These results support the recruitment model of transcriptional activation and, combined with other studies, suggest the possibility of using activator peptides in a variety of applications, including drug development work.
Figures




References
LinkOut - more resources
Full Text Sources

