Pivotal role of VASP in Arp2/3 complex-mediated actin nucleation, actin branch-formation, and Listeria monocytogenes motility
- PMID: 11581288
- PMCID: PMC2150787
- DOI: 10.1083/jcb.200106061
Pivotal role of VASP in Arp2/3 complex-mediated actin nucleation, actin branch-formation, and Listeria monocytogenes motility
Abstract
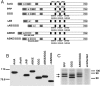
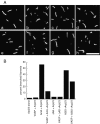
The Listeria monocytogenes ActA protein mediates actin-based motility by recruiting and stimulating the Arp2/3 complex. In vitro, the actin monomer-binding region of ActA is critical for stimulating Arp2/3-dependent actin nucleation; however, this region is dispensable for actin-based motility in cells. Here, we provide genetic and biochemical evidence that vasodilator-stimulated phosphoprotein (VASP) recruitment by ActA can bypass defects in actin monomer-binding. Furthermore, purified VASP enhances the actin-nucleating activity of wild-type ActA and the Arp2/3 complex while also reducing the frequency of actin branch formation. These data suggest that ActA stimulates the Arp2/3 complex by both VASP-dependent and -independent mechanisms that generate distinct populations of actin filaments in the comet tails of L. monocytogenes. The ability of VASP to contribute to actin filament nucleation and to regulate actin filament architecture highlights the central role of VASP in actin-based motility.
Figures





Similar articles
-
Adapter protein SH2-Bbeta stimulates actin-based motility of Listeria monocytogenes in a vasodilator-stimulated phosphoprotein (VASP)-dependent fashion.Infect Immun. 2007 Jul;75(7):3581-93. doi: 10.1128/IAI.00214-07. Epub 2007 Apr 23. Infect Immun. 2007. PMID: 17452473 Free PMC article.
-
The bacterial actin nucleator protein ActA of Listeria monocytogenes contains multiple binding sites for host microfilament proteins.Curr Biol. 1995 May 1;5(5):517-25. doi: 10.1016/s0960-9822(95)00104-7. Curr Biol. 1995. PMID: 7583101
-
Identification of cofilin, coronin, Rac and capZ in actin tails using a Listeria affinity approach.J Cell Sci. 1998 Oct;111 ( Pt 19):2877-84. doi: 10.1242/jcs.111.19.2877. J Cell Sci. 1998. PMID: 9730980
-
Actin-based motility as a self-organized system: mechanism and reconstitution in vitro.C R Biol. 2003 Feb;326(2):161-70. doi: 10.1016/s1631-0691(03)00067-2. C R Biol. 2003. PMID: 12754935 Review.
-
Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration.Annu Rev Cell Dev Biol. 2003;19:541-64. doi: 10.1146/annurev.cellbio.19.050103.103356. Annu Rev Cell Dev Biol. 2003. PMID: 14570581 Review.
Cited by
-
Ena/VASP proteins can regulate distinct modes of actin organization at cadherin-adhesive contacts.Mol Biol Cell. 2006 Mar;17(3):1085-95. doi: 10.1091/mbc.e05-07-0644. Epub 2005 Dec 21. Mol Biol Cell. 2006. PMID: 16371509 Free PMC article.
-
The receptor protein tyrosine phosphatase PTP69D antagonizes Abl tyrosine kinase to guide axons in Drosophila.Mech Dev. 2008 Mar-Apr;125(3-4):247-56. doi: 10.1016/j.mod.2007.11.005. Epub 2007 Nov 22. Mech Dev. 2008. PMID: 18160268 Free PMC article.
-
Cooperation of Striatin 3 and MAP4K4 promotes growth and tissue invasion.Commun Biol. 2022 Aug 8;5(1):795. doi: 10.1038/s42003-022-03708-y. Commun Biol. 2022. PMID: 35941177 Free PMC article.
-
Profilin Interaction with Actin Filament Barbed End Controls Dynamic Instability, Capping, Branching, and Motility.Dev Cell. 2016 Jan 25;36(2):201-14. doi: 10.1016/j.devcel.2015.12.024. Dev Cell. 2016. PMID: 26812019 Free PMC article.
-
Contribution of Ena/VASP proteins to intracellular motility of listeria requires phosphorylation and proline-rich core but not F-actin binding or multimerization.Mol Biol Cell. 2002 Jul;13(7):2383-96. doi: 10.1091/mbc.e02-01-0058. Mol Biol Cell. 2002. PMID: 12134077 Free PMC article.
References
-
- Amann, K.J., and T.D. Pollard. 2001. The Arp2/3 complex nucleates actin filament branches from the sides of pre-existing filaments. Nat. Cell Biol. 3:306–310. - PubMed
-
- Bachmann, C., L. Fischer, U. Walter, and M. Reinhard. 1999. The EVH2 domain of the vasodilator-stimulated phosphoprotein mediates tetramerization, F-actin binding, and actin bundle formation. J. Biol. Chem. 274:23549–23557. - PubMed
-
- Bear, J.E., J.J. Loureiro, I. Libova, R. Fassler, J. Wehland, and F.B. Gertler. 2000. Negative regulation of fibroblast motility by Ena/VASP proteins. Cell. 101:717–728. - PubMed
-
- Bi, E., and S.H. Zigmond. 1999. Actin polymerization: Where the WASP stings. Curr. Biol. 9:R160–R163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

