Mitochondrial coupling factor 6 as a potent endogenous vasoconstrictor
- PMID: 11581303
- PMCID: PMC200946
- DOI: 10.1172/JCI11076
Mitochondrial coupling factor 6 as a potent endogenous vasoconstrictor
Abstract
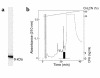
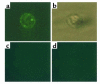
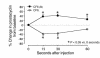
We demonstrated recently that coupling factor 6, an essential component of the energy-transducing stalk of mitochondrial ATP synthase, suppresses the synthesis of prostacyclin in vascular endothelial cells. Here, we tested the hypothesis that coupling factor 6 is present on the cell surface and is involved in the regulation of systemic circulation. This peptide is present on the surface of CRL-2222 vascular endothelial cells and is released by these cells into the medium. In vivo, the peptide circulates in the vascular system of the rat, and its gene expression and plasma concentration are higher in spontaneously hypertensive rats (SHRs) than in normotensive controls. Elevation of blood pressure with norepinephrine did not affect the plasma concentration of coupling factor 6. Intravenous injection of recombinant peptide increased blood pressure, apparently by suppressing prostacyclin synthesis, whereas a specific Ab to coupling factor 6 decreased systemic blood pressure concomitantly with an increase in plasma prostacyclin. Interestingly, the antibody's hypotensive effect could be abolished by treating with the cyclooxygenase inhibitor indomethacin. These findings indicate that mitochondrial coupling factor 6 functions as a potent endogenous vasoconstrictor in the fashion of a circulating hormone and may suggest a new mechanism for hypertension.
Figures
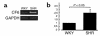
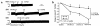
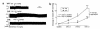
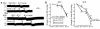
Similar articles
-
Coupling factor 6-induced prostacyclin inhibition is enhanced in vascular smooth muscle cells from spontaneously hypertensive rats.J Hypertens. 2009 Sep;27(9):1823-8. doi: 10.1097/HJH.0b013e32832d4b05. J Hypertens. 2009. PMID: 19474762
-
A novel inhibitory effect on prostacyclin synthesis of coupling factor 6 extracted from the heart of spontaneously hypertensive rats.J Biol Chem. 1998 Nov 27;273(48):31778-83. doi: 10.1074/jbc.273.48.31778. J Biol Chem. 1998. PMID: 9822642
-
Coupling factor 6 enhances Src-mediated responsiveness to angiotensin II in resistance arterioles and cells.Cardiovasc Res. 2009 Mar 1;81(4):780-7. doi: 10.1093/cvr/cvn356. Epub 2008 Dec 22. Cardiovasc Res. 2009. PMID: 19106112
-
Coupling factor 6 as a novel vasoactive and proatherogenic peptide in vascular endothelial cells.Naunyn Schmiedebergs Arch Pharmacol. 2009 Sep;380(3):205-14. doi: 10.1007/s00210-009-0431-y. Epub 2009 Jun 2. Naunyn Schmiedebergs Arch Pharmacol. 2009. PMID: 19488738 Review.
-
Recent advances in structure-functional studies of mitochondrial factor B.J Bioenerg Biomembr. 2009 Apr;41(2):137-43. doi: 10.1007/s10863-009-9210-1. J Bioenerg Biomembr. 2009. PMID: 19377834 Free PMC article. Review.
Cited by
-
Whole-Blood 3-Gene Signature as a Decision Aid for Rifapentine-based Tuberculosis Preventive Therapy.Clin Infect Dis. 2022 Sep 14;75(5):743-752. doi: 10.1093/cid/ciac003. Clin Infect Dis. 2022. PMID: 34989801 Free PMC article.
-
Marine collagen peptides reduce endothelial cell injury in diabetic rats by inhibiting apoptosis and the expression of coupling factor 6 and microparticles.Mol Med Rep. 2017 Oct;16(4):3947-3957. doi: 10.3892/mmr.2017.7061. Epub 2017 Jul 21. Mol Med Rep. 2017. PMID: 28731155 Free PMC article.
-
Pathogenesis of cardiovascular diseases: effects of mitochondrial CF6 on endothelial cell function.Mol Cell Biochem. 2025 Feb;480(2):841-853. doi: 10.1007/s11010-024-05065-2. Epub 2024 Jul 10. Mol Cell Biochem. 2025. PMID: 38985252 Review.
-
Physiological Consequences of Coronary Arteriolar Dysfunction and Its Influence on Cardiovascular Disease.Physiology (Bethesda). 2018 Sep 1;33(5):338-347. doi: 10.1152/physiol.00019.2018. Physiology (Bethesda). 2018. PMID: 30109826 Free PMC article. Review.
-
Regulation of endothelial function by mitochondrial reactive oxygen species.Antioxid Redox Signal. 2011 Sep 15;15(6):1517-30. doi: 10.1089/ars.2010.3642. Epub 2011 Apr 26. Antioxid Redox Signal. 2011. PMID: 21194353 Free PMC article. Review.
References
-
- Boyer PD. The binding change mechanism for ATP synthase: some probabilities and possibilities. Biochim Biophys Acta. 1993;1140:215–250. - PubMed
-
- Walker JE, et al. Primary structure and subunit stoichiometry of F1-ATPase from bovine mitochondria. J Mol Biol. 1985;184:677–701. - PubMed
-
- Kagawa Y, Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. Correlation of morphology and function in submitochondrial particles. J Biol Chem. 1966;241:2475–2482. - PubMed
-
- Walker JE, Runswick MJ, Poulter L. ATP synthase from bovine mitochondria: characterization and sequence analysis of two membrane associated subunits and of their corresponding c-DNAs. J Mol Biol. 1987;197:89–100. - PubMed
-
- Collinson IR, et al. ATP synthase from bovine heart mitochondria. In vitro assembly of a stalk complex in the presence of F1-ATPase and its absence. J Mol Biol. 1994;242:408–421. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

