Mitochondrial coupling factor 6 as a potent endogenous vasoconstrictor
- PMID: 11581303
- PMCID: PMC200946
- DOI: 10.1172/JCI11076
Mitochondrial coupling factor 6 as a potent endogenous vasoconstrictor
Abstract
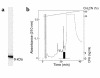
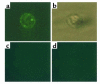
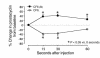
We demonstrated recently that coupling factor 6, an essential component of the energy-transducing stalk of mitochondrial ATP synthase, suppresses the synthesis of prostacyclin in vascular endothelial cells. Here, we tested the hypothesis that coupling factor 6 is present on the cell surface and is involved in the regulation of systemic circulation. This peptide is present on the surface of CRL-2222 vascular endothelial cells and is released by these cells into the medium. In vivo, the peptide circulates in the vascular system of the rat, and its gene expression and plasma concentration are higher in spontaneously hypertensive rats (SHRs) than in normotensive controls. Elevation of blood pressure with norepinephrine did not affect the plasma concentration of coupling factor 6. Intravenous injection of recombinant peptide increased blood pressure, apparently by suppressing prostacyclin synthesis, whereas a specific Ab to coupling factor 6 decreased systemic blood pressure concomitantly with an increase in plasma prostacyclin. Interestingly, the antibody's hypotensive effect could be abolished by treating with the cyclooxygenase inhibitor indomethacin. These findings indicate that mitochondrial coupling factor 6 functions as a potent endogenous vasoconstrictor in the fashion of a circulating hormone and may suggest a new mechanism for hypertension.
Figures
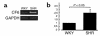
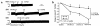
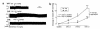
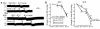
References
-
- Boyer PD. The binding change mechanism for ATP synthase: some probabilities and possibilities. Biochim Biophys Acta. 1993;1140:215–250. - PubMed
-
- Walker JE, et al. Primary structure and subunit stoichiometry of F1-ATPase from bovine mitochondria. J Mol Biol. 1985;184:677–701. - PubMed
-
- Kagawa Y, Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. Correlation of morphology and function in submitochondrial particles. J Biol Chem. 1966;241:2475–2482. - PubMed
-
- Walker JE, Runswick MJ, Poulter L. ATP synthase from bovine mitochondria: characterization and sequence analysis of two membrane associated subunits and of their corresponding c-DNAs. J Mol Biol. 1987;197:89–100. - PubMed
-
- Collinson IR, et al. ATP synthase from bovine heart mitochondria. In vitro assembly of a stalk complex in the presence of F1-ATPase and its absence. J Mol Biol. 1994;242:408–421. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

