Inhibition of IgE-mediated mast cell activation by the paired Ig-like receptor PIR-B
- PMID: 11581305
- PMCID: PMC200947
- DOI: 10.1172/JCI12195
Inhibition of IgE-mediated mast cell activation by the paired Ig-like receptor PIR-B
Abstract
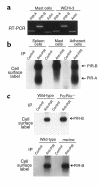
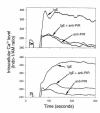
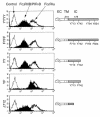
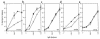
The potential of the paired Ig-like receptors of activating (PIR-A) and inhibitory (PIR-B) types for modifying an IgE antibody-mediated allergic response was evaluated in mouse bone marrow-derived mast cells. Although mast cells produced both PIR-A and PIR-B, PIR-B was found to be preferentially expressed on the cell surface, where it was constitutively tyrosine phosphorylated and associated with intracellular SHP-1 protein tyrosine phosphatase. PIR-B coligation with the IgE receptor (FcepsilonRI) inhibited IgE-mediated mast cell activation and release of serotonin. Surprisingly, the inhibitory activity of PIR-B was unimpaired in SHP-1-deficient mast cells. A third functional tyrosine-based inhibitory motif, one that fails to bind the SHP-1, SHP-2, and SHIP phosphatases, was identified in parallel studies of FcepsilonRI-bearing rat basophilic leukemia (RBL) cells transfected with constructs having mutations in the PIR-B cytoplasmic region. These results define the preferential expression of the PIR-B molecules on mast cells and an inhibitory potential that can be mediated via a SHP-1-independent pathway.
Figures



Similar articles
-
Mast cell regulation via paired immunoglobulin-like receptor PIR-B.Immunol Res. 2002;26(1-3):191-7. doi: 10.1385/ir:26:1-3:191. Immunol Res. 2002. PMID: 12403357 Review.
-
gp49B1 inhibits IgE-initiated mast cell activation through both immunoreceptor tyrosine-based inhibitory motifs, recruitment of src homology 2 domain-containing phosphatase-1, and suppression of early and late calcium mobilization.J Biol Chem. 1999 Feb 26;274(9):5791-6. doi: 10.1074/jbc.274.9.5791. J Biol Chem. 1999. PMID: 10026201
-
Selective in vivo recruitment of the phosphatidylinositol phosphatase SHIP by phosphorylated Fc gammaRIIB during negative regulation of IgE-dependent mouse mast cell activation.Immunol Lett. 1996 Dec;54(2-3):83-91. doi: 10.1016/s0165-2478(96)02654-5. Immunol Lett. 1996. PMID: 9052859
-
Requirement of the tyrosines at residues 258 and 270 of MAIR-I in inhibitory effect on degranulation from basophilic leukemia RBL-2H3.Int Immunol. 2005 Jan;17(1):65-72. doi: 10.1093/intimm/dxh187. Epub 2004 Nov 29. Int Immunol. 2005. PMID: 15569773
-
[Mast cell inhibitory receptors].Postepy Hig Med Dosw (Online). 2012 Oct 22;66:739-51. doi: 10.5604/17322693.1015039. Postepy Hig Med Dosw (Online). 2012. PMID: 23175328 Review. Polish.
Cited by
-
Ly49Q, a member of the Ly49 family that is selectively expressed on myeloid lineage cells and involved in regulation of cytoskeletal architecture.Proc Natl Acad Sci U S A. 2004 Jan 27;101(4):1016-21. doi: 10.1073/pnas.0305400101. Epub 2004 Jan 19. Proc Natl Acad Sci U S A. 2004. PMID: 14732700 Free PMC article.
-
Paired immunoglobulin-like receptors and their MHC class I recognition.Immunology. 2005 Aug;115(4):433-40. doi: 10.1111/j.1365-2567.2005.02177.x. Immunology. 2005. PMID: 16011512 Free PMC article. Review.
-
Role of PIR-B in autoimmune glomerulonephritis.J Biomed Biotechnol. 2011;2011:275302. doi: 10.1155/2011/275302. Epub 2010 Oct 4. J Biomed Biotechnol. 2011. PMID: 20976309 Free PMC article. Review.
-
Cell surface signaling molecules in the control of immune responses: a tide model.Immunity. 2011 Apr 22;34(4):466-78. doi: 10.1016/j.immuni.2011.04.008. Immunity. 2011. PMID: 21511182 Free PMC article. Review.
-
Substitution of herpes simplex virus 1 entry glycoproteins with those of saimiriine herpesvirus 1 reveals a gD-gH/gL functional interaction and a region within the gD profusion domain that is critical for fusion.J Virol. 2014 Jun;88(11):6470-82. doi: 10.1128/JVI.00465-14. Epub 2014 Mar 26. J Virol. 2014. PMID: 24672037 Free PMC article.
References
-
- Hayami KD, et al. Molecular cloning of a novel murine cell-surface glycoprotein homologous to killer cell inhibitory receptors. J Biol Chem. 1997;272:7320–7327. - PubMed
-
- Samaridis J, Colonna M. Cloning of novel immunoglobulin superfamily receptors expressed on human myeloid and lymphoid cells: structural evidence for new stimulatory and inhibitory pathways. Eur J Immunol. 1997;27:660–665. - PubMed
-
- Cosman D, et al. A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity. 1997;7:273–282. - PubMed
-
- Wagtmann N, Rojo S, Eichler E, Mohrenweiser H, Long EO. A new human gene complex encoding the killer cells inhibitory receptors and related monocyte/macrophage receptors. Curr Biol. 1997;7:615–618. - PubMed

