Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis
- PMID: 11581310
- PMCID: PMC2193476
- DOI: 10.1084/jem.194.7.873
Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis
Abstract
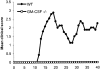
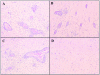
Experimental autoimmune encephalomyelitis (EAE), a model for multiple sclerosis, can be induced by immunization with a number of myelin antigens. In particular, myelin oligodendrocyte glycoprotein, a central nervous system (CNS)-specific antigen expressed on the myelin surface, is able to induce a paralytic MS-like disease with extensive CNS inflammation and demyelination in several strains of animals. Although not well understood, the egress of immune cells into the CNS in EAE is governed by a complex interplay between pro and antiinflammatory cytokines and chemokines. The hematopoietic growth factor, granulocyte macrophage colony-stimulating factor (GM-CSF), is considered to play a central role in maintaining chronic inflammation. The present study was designed to investigate the previously unexplored role of GM-CSF in autoimmune-mediated demyelination. GM-CSF(-/)- mice are resistant to EAE, display decreased antigen-specific proliferation of splenocytes, and fail to sustain immune cell infiltrates in the CNS, thus revealing key activities for GM-CSF in the development of inflammatory demyelinating lesions and control of migration and/or proliferation of leukocytes within the CNS. These results hold implications for the pathogenesis of inflammatory and demyelinating diseases and may provide the basis for more effective therapies for inflammatory diseases, and more specifically for multiple sclerosis.
Figures







Similar articles
-
GM-CSF Promotes Chronic Disability in Experimental Autoimmune Encephalomyelitis by Altering the Composition of Central Nervous System-Infiltrating Cells, but Is Dispensable for Disease Induction.J Immunol. 2018 Feb 1;200(3):966-973. doi: 10.4049/jimmunol.1701484. Epub 2017 Dec 29. J Immunol. 2018. PMID: 29288202 Free PMC article.
-
Fc receptors are critical for autoimmune inflammatory damage to the central nervous system in experimental autoimmune encephalomyelitis.Scand J Immunol. 2002 Jan;55(1):70-81. doi: 10.1046/j.1365-3083.2002.01024.x. Scand J Immunol. 2002. PMID: 11841694
-
Targeting the GM-CSF receptor for the treatment of CNS autoimmunity.J Autoimmun. 2017 Nov;84:1-11. doi: 10.1016/j.jaut.2017.06.005. Epub 2017 Jun 20. J Autoimmun. 2017. PMID: 28641926 Free PMC article.
-
The Role of Granulocyte-Macrophage Colony-Stimulating Factor in Murine Models of Multiple Sclerosis.Cells. 2020 Mar 4;9(3):611. doi: 10.3390/cells9030611. Cells. 2020. PMID: 32143326 Free PMC article. Review.
-
Immune responses against the myelin/oligodendrocyte glycoprotein in experimental autoimmune demyelination.J Clin Immunol. 2001 May;21(3):155-70. doi: 10.1023/a:1011031014433. J Clin Immunol. 2001. PMID: 11403222 Review.
Cited by
-
The IL-27/IL-27R axis is altered in CD4+ and CD8+ T lymphocytes from multiple sclerosis patients.Clin Transl Immunology. 2021 Mar 5;10(3):e1262. doi: 10.1002/cti2.1262. eCollection 2021. Clin Transl Immunology. 2021. PMID: 33728050 Free PMC article.
-
Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage.J Leukoc Biol. 2016 Sep;100(3):481-9. doi: 10.1189/jlb.3RU0316-144R. Epub 2016 Jun 28. J Leukoc Biol. 2016. PMID: 27354413 Free PMC article. Review.
-
Pertussis Toxin Inhibits Encephalitogenic T-Cell Infiltration and Promotes a B-Cell-Driven Disease during Th17-EAE.Int J Mol Sci. 2021 Mar 13;22(6):2924. doi: 10.3390/ijms22062924. Int J Mol Sci. 2021. PMID: 33805762 Free PMC article.
-
Limiting multiple sclerosis related axonopathy by blocking Nogo receptor and CRMP-2 phosphorylation.Brain. 2012 Jun;135(Pt 6):1794-818. doi: 10.1093/brain/aws100. Epub 2012 Apr 28. Brain. 2012. PMID: 22544872 Free PMC article.
-
Fumaric acid esters are effective in chronic experimental autoimmune encephalomyelitis and suppress macrophage infiltration.Clin Exp Immunol. 2006 Jul;145(1):101-7. doi: 10.1111/j.1365-2249.2006.03094.x. Clin Exp Immunol. 2006. PMID: 16792679 Free PMC article.
References
-
- Steinman L. Assessment of animal models for MS and demyelinating disease in the design of rational therapy. Neuron. 1999;24:511–514. - PubMed
-
- Sobel R.A., Greer J.M., Kuchroo V.K. Minireviewautoimmune responses to myelin proteolipid protein. Neurochem. Res. 1994;19:915–921. - PubMed
-
- Bernard C.C.A., Ichikawa M., Menon K., Slavin A., Ewing C., Johns T., Liu J., Bettadapura J. Autoantigens in experimental autoimmune encephalomyelitis and multiple sclerosis Abramsky O., Ovadia H. Frontiers in Multiple SclerosisClinical Research and Therapy 1997. 61 70 Martin Dunitz Limited; London: pp
-
- Bernard C.C., Johns T.G., Slavin A., Ichikawa M., Ewing C., Liu J., Bettadapura J. Myelin oligodendrocyte glycoproteina novel candidate autoantigen in multiple sclerosis. J. Mol. Med. 1997;75:77–88. - PubMed
-
- Kerlero de Rosbo N., Brok H.P., Bauer J., Kaye J.F., Hart B.A., Ben-Nun A. Rhesus monkeys are highly susceptible to experimental autoimmune encephalomyelitis induced by myelin oligodendrocyte glycoproteincharacterization of immunodominant T- and B-cell epitopes. J. Neuroimmunol. 2000;110:83–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

