Notch 1-deficient common lymphoid precursors adopt a B cell fate in the thymus
- PMID: 11581321
- PMCID: PMC2193487
- DOI: 10.1084/jem.194.7.1003
Notch 1-deficient common lymphoid precursors adopt a B cell fate in the thymus
Abstract
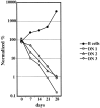
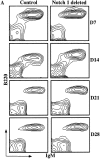
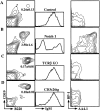
We have recently reported that Notch 1, a member of the Notch multigene family, is essential for the development of murine T cells. Using a mouse model in which Notch 1 is inactivated in bone marrow (BM) precursors we have shown that B cells instead of T cells are found in the thymus of BM chimeras. However, it is not clear whether these B cells develop by default from a common lymphoid precursor due to the absence of Notch 1 signaling, or whether they arise as a result of perturbed migration of BM-derived B cells and/or altered homeostasis of normal resident thymic B cells. In this report we show that Notch 1-deficient thymic B cells resemble BM B cells in phenotype and turnover kinetics and are located predominantly in the medulla and corticomedullary junction. Peripheral blood lymphocyte analysis shows no evidence of recirculating Notch1(-/)- BM B cells. Furthermore, lack of T cell development is not due to a failure of Notch1(-/)- precursors to home to the thymus, as even after intrathymic reconstitution with BM cells, B cells instead of T cells develop from Notch 1-deficient precursors. Taken together, these results provide evidence for de novo ectopic B cell development in the thymus, and support the hypothesis that in the absence of Notch 1 common lymphoid precursors adopt the default cell fate and develop into B cells instead.
Figures







Comment in
-
Coming to grips with Notch.J Exp Med. 2001 Oct 1;194(7):F43-6. doi: 10.1084/jem.194.7.f43. J Exp Med. 2001. PMID: 11581323 Free PMC article. No abstract available.
References
-
- Artavanis-Tsakonas S., Rand M.D., Lake R.J. Notch signalingcell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
-
- Artavanis-Tsakonas S., Matsuno K., Fortini M.E. Notch signaling. Science. 1995;268:225–232. - PubMed
-
- Osborne B., Miele L. Notch and the immune system. Immunity. 1999;11:653–663. - PubMed
-
- Robey E. Regulation of T cell fate by Notch. Annu. Rev. Immunol. 1999;17:283–295. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

