Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity
- PMID: 11581376
- PMCID: PMC114582
- DOI: 10.1128/JVI.75.21.10073-10089.2001
Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity
Abstract
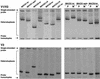
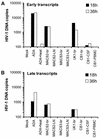
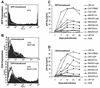
The viral determinants that underlie human immunodeficiency virus type 1 (HIV-1) neurotropism are unknown, due in part to limited studies on viruses isolated from brain. Previous studies suggest that brain-derived viruses are macrophage tropic (M-tropic) and principally use CCR5 for virus entry. To better understand HIV-1 neurotropism, we isolated primary viruses from autopsy brain, cerebral spinal fluid, blood, spleen, and lymph node samples from AIDS patients with dementia and HIV-1 encephalitis. Isolates were characterized to determine coreceptor usage and replication capacity in peripheral blood mononuclear cells (PBMC), monocyte-derived macrophages (MDM), and microglia. Env V1/V2 and V3 heteroduplex tracking assay and sequence analyses were performed to characterize distinct variants in viral quasispecies. Viruses isolated from brain, which consisted of variants that were distinct from those in lymphoid tissues, used CCR5 (R5), CXCR4 (X4), or both coreceptors (R5X4). Minor usage of CCR2b, CCR3, CCR8, and Apj was also observed. Primary brain and lymphoid isolates that replicated to high levels in MDM showed a similar capacity to replicate in microglia. Six of 11 R5 isolates that replicated efficiently in PBMC could not replicate in MDM or microglia due to a block in virus entry. CD4 overexpression in microglia transduced with retroviral vectors had no effect on the restricted replication of these virus strains. Furthermore, infection of transfected cells expressing different amounts of CD4 or CCR5 with M-tropic and non-M-tropic R5 isolates revealed a similar dependence on CD4 and CCR5 levels for entry, suggesting that the entry block was not due to low levels of either receptor. Studies using TAK-779 and AMD3100 showed that two highly M-tropic isolates entered microglia primarily via CXCR4. These results suggest that HIV-1 tropism for macrophages and microglia is restricted at the entry level by a mechanism independent of coreceptor specificity. These findings provide evidence that M-tropism rather than CCR5 usage predicts HIV-1 neurotropism.
Figures



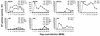



References
-
- Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
-
- Balliet J W, Kolson D L, Eiger G, Kim F M, McGann K A, Srinivasan A, Collman R. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology. 1994;200:623–631. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS35734/NS/NINDS NIH HHS/United States
- P30 AI028691/AI/NIAID NIH HHS/United States
- T32-AI07419/AI/NIAID NIH HHS/United States
- R01 NS035734/NS/NINDS NIH HHS/United States
- AI28697/AI/NIAID NIH HHS/United States
- R01 AI036554/AI/NIAID NIH HHS/United States
- AI36059/AI/NIAID NIH HHS/United States
- CA79458/CA/NCI NIH HHS/United States
- P30 AI028697/AI/NIAID NIH HHS/United States
- R01 NS037277/NS/NINDS NIH HHS/United States
- R37 AI044667/AI/NIAID NIH HHS/United States
- AI28691/AI/NIAID NIH HHS/United States
- R01 AI044667/AI/NIAID NIH HHS/United States
- R37 AI036059/AI/NIAID NIH HHS/United States
- AI36554/AI/NIAID NIH HHS/United States
- R56 AI044667/AI/NIAID NIH HHS/United States
- T32 AI007419/AI/NIAID NIH HHS/United States
- AI44667/AI/NIAID NIH HHS/United States
- HD37260/HD/NICHD NIH HHS/United States
- DA13127/DA/NIDA NIH HHS/United States
- NS37277/NS/NINDS NIH HHS/United States
- U01 AI35039/AI/NIAID NIH HHS/United States
- U01 AI035039/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

