Neutralizing antibodies associated with viremia control in a subset of individuals after treatment of acute human immunodeficiency virus type 1 infection
- PMID: 11581388
- PMCID: PMC114594
- DOI: 10.1128/JVI.75.21.10200-10207.2001
Neutralizing antibodies associated with viremia control in a subset of individuals after treatment of acute human immunodeficiency virus type 1 infection
Abstract
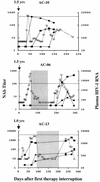
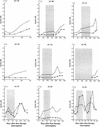
Immediate treatment of acute human immunodeficiency virus type 1 (HIV-1) infection has been associated with subsequent control of viremia in a subset of patients after therapy cessation, but the immune responses contributing to control have not been fully defined. Here we examined neutralizing antibodies as a correlate of viremia control following treatment interruption in HIV-1-infected individuals in whom highly active antiretriviral therapy (HAART) was initiated during early seroconversion and who remained on therapy for 1 to 3 years. Immediately following treatment interruption, neutralizing antibodies were undetectable with T-cell-line adapted strains and the autologous primary HIV-1 isolate in seven of nine subjects. Env- and Gag-specific antibodies as measured by enzyme-linked immunosorbent assay were also low or undetectable at this time. Despite this apparent poor maturation of the virus-specific B-cell response during HAART, autologous neutralizing antibodies emerged rapidly and correlated with a spontaneous downregulation in rebound viremia following treatment interruption in three subjects. Control of rebound viremia was seen in other subjects in the absence of detectable neutralizing antibodies. The results indicate that virus-specific B-cell priming occurs despite the early institution of HAART, allowing rapid secondary neutralizing-antibody production following treatment interruption in a subset of individuals. Since early HAART limits viral diversification, we hypothesize that potent neutralizing-antibody responses to autologous virus are able to mature and that in some persons these responses contribute to the control of plasma viremia after treatment cessation.
Figures


References
-
- Altfeld M, Rosenberg E S, Shankarappa R, Mukherjee J S, Hecht F M, Eldridge R L, Addo M M, Poon S H, Phillips M N, Robbins G K, Sax P E, Boswell S, Kahn J O, Brander C, Goulder P J R, Levy J A, Mullins J I, Walker B D. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J Exp Med. 2001;193:169–180. - PMC - PubMed
-
- Ariyoshi K, Harwood E, Chiengsong-Popov R, Weber J. Is clearance of HIV-1 viremia at seroconversion mediated by neutralizing antibodies? Lancet. 1992;340:1257–1258. - PubMed
-
- Autran B, Carcelain G, Li T S, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. - PubMed
-
- Berger E A, Doms R W, Fenyö E M, Korber B T M, Littman D R, Moore J P, Sattentau Q J, Schuitemaker H, Sodroski J, Weiss R A. HIV-1 phenotypes classified by co-receptor usage. Nature. 1998;391:240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

