The synthetic peptide P-197 inhibits human T-cell leukemia virus type 1 envelope-mediated syncytium formation by a mechanism that is independent of Hsc70
- PMID: 11581416
- PMCID: PMC114622
- DOI: 10.1128/JVI.75.21.10472-10478.2001
The synthetic peptide P-197 inhibits human T-cell leukemia virus type 1 envelope-mediated syncytium formation by a mechanism that is independent of Hsc70
Abstract
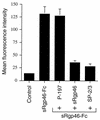
Entry of human T-cell leukemia virus type 1 (HTLV-1) into cells is mediated by the viral envelope glycoproteins gp46 and gp21. The gp46 surface glycoprotein binds to a poorly characterized cell surface receptor, thereby promoting the gp21-dependent fusion of the viral and cellular membranes. Interestingly, a synthetic peptide (P-197) simulating amino acids 197 to 216 of gp46 strongly inhibits envelope-dependent membrane fusion with Molt-4 target cells. It has been suggested that this peptide acts by competitively binding to Hsc70, a putative cellular receptor for HTLV-1. We now demonstrate that P-197 inhibits membrane fusion among diverse HTLV-1-permissive target cells. Importantly, most of these cells lack detectable levels of Hsc70, indicating that P-197 inhibits membrane fusion by a mechanism that is Hsc70 independent. We now suggest that competition for primary receptor binding is unlikely to account for the inhibitory activity of P-197. Understanding the mechanism by which P-197 functions may reveal concepts of general relevance to antiretroviral chemotherapy.
Figures




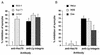
References
-
- Cann A J, Chen I S Y. Human T-cell leukemia virus type I and II. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1501–1527.
-
- Daenke S, McCracken S A, Booth S. Human T-cell leukaemia/lymphoma virus type 1 syncytium formation is regulated in a cell-specific manner by ICAM-1, ICAM-3 and VCAM-1 and can be inhibited by antibodies to integrin β2 or β7. J Gen Virol. 1999;80:1429–1436. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

